Comprehension # 8
`A` factory, producing methanol, is based on the reaction:
`CO+2H_(2)hArrCH_(3)OH`
Hydrogen `&` carbon monoxide are obtained by the reaction
`CH_(4)+H_(2)OhArrCO+3H_(2)`
Three units of factory namely, the "reformer" for the `H_(2)` and `CO` production, the "methanol reactor" for production of methanol and a "separator" to separate `CH_(3)OH` from `CO` and `H_(2)` are schematically shown in figure.
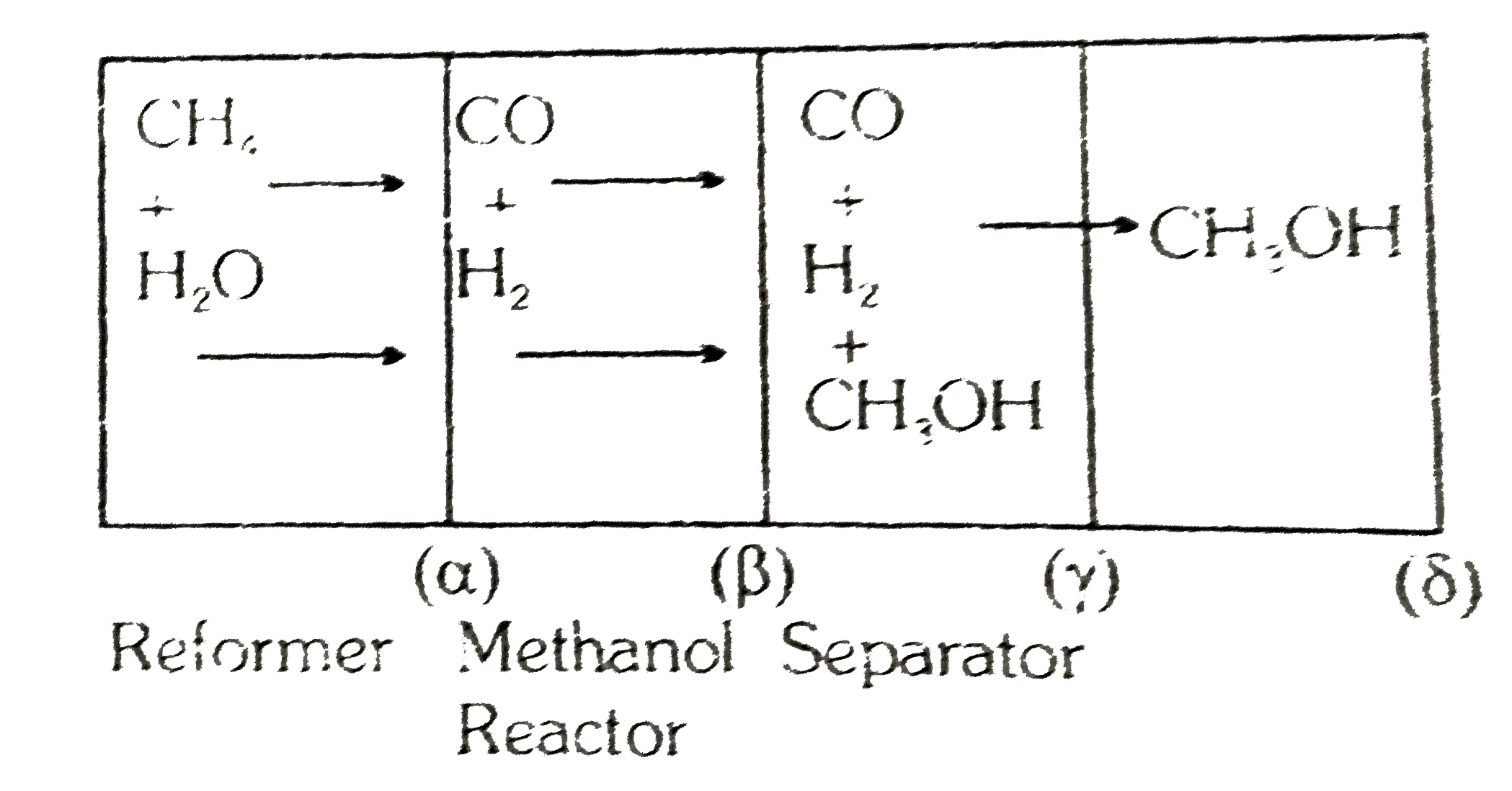
four positions are indicated as `alpha,beta,gamma` and `delta`. The flow of methanol at position `gamma` is `10^(3)mol//sec`. The factory is so designed that `(2)/(3)` of the `CO` is converted to `CH_(3)OH`. Excess of `CO` and `H_(2)` at position `delta` are used to heat the first reaction. Assume that the reformer reaction goes to completion. At the position `(beta)` mole ratio of `CO` to `H_(2)` is `(1)/(3)`
`CO+2H_(2)hArrCH_(3)OH " "DeltaH_(r)=-100 R`
What is the flow of `CO` and `H_(2)` at position `(beta)` ?