A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-MOLE CONCEPT-Comprehension # 9
- Comprehension # 9 A 10ml mixture of N(2), a alkane & O(2) undergo co...
Text Solution
|
- Comprehension # 9 A 10ml mixture of N(2), a alkane & O(2) undergo co...
Text Solution
|
- Comprehension # 9 A 10ml mixture of N(2), a alkane & O(2) undergo co...
Text Solution
|
- Comprehension # 9 A 10ml mixture of N(2), a alkane & O(2) undergo co...
Text Solution
|
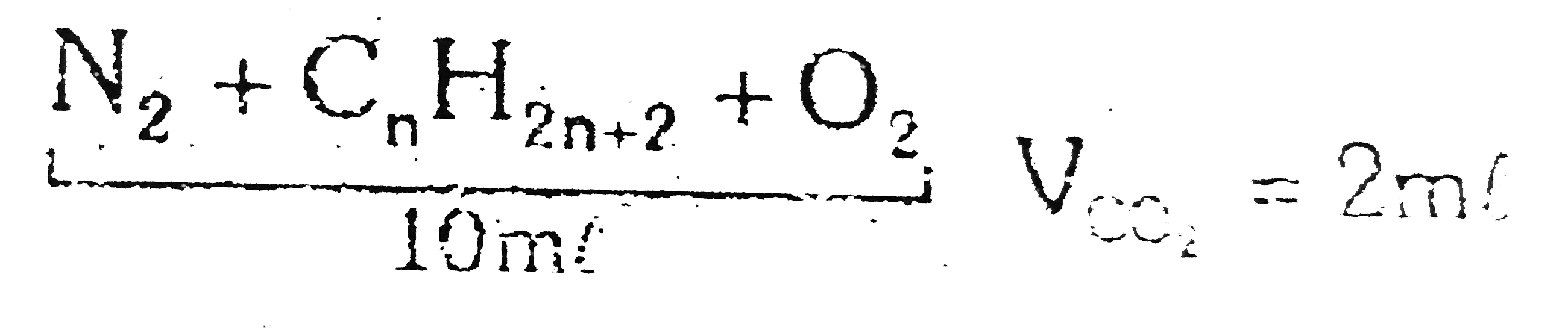 `V_(CO_(2)) = 2ml`
`V_(CO_(2)) = 2ml`