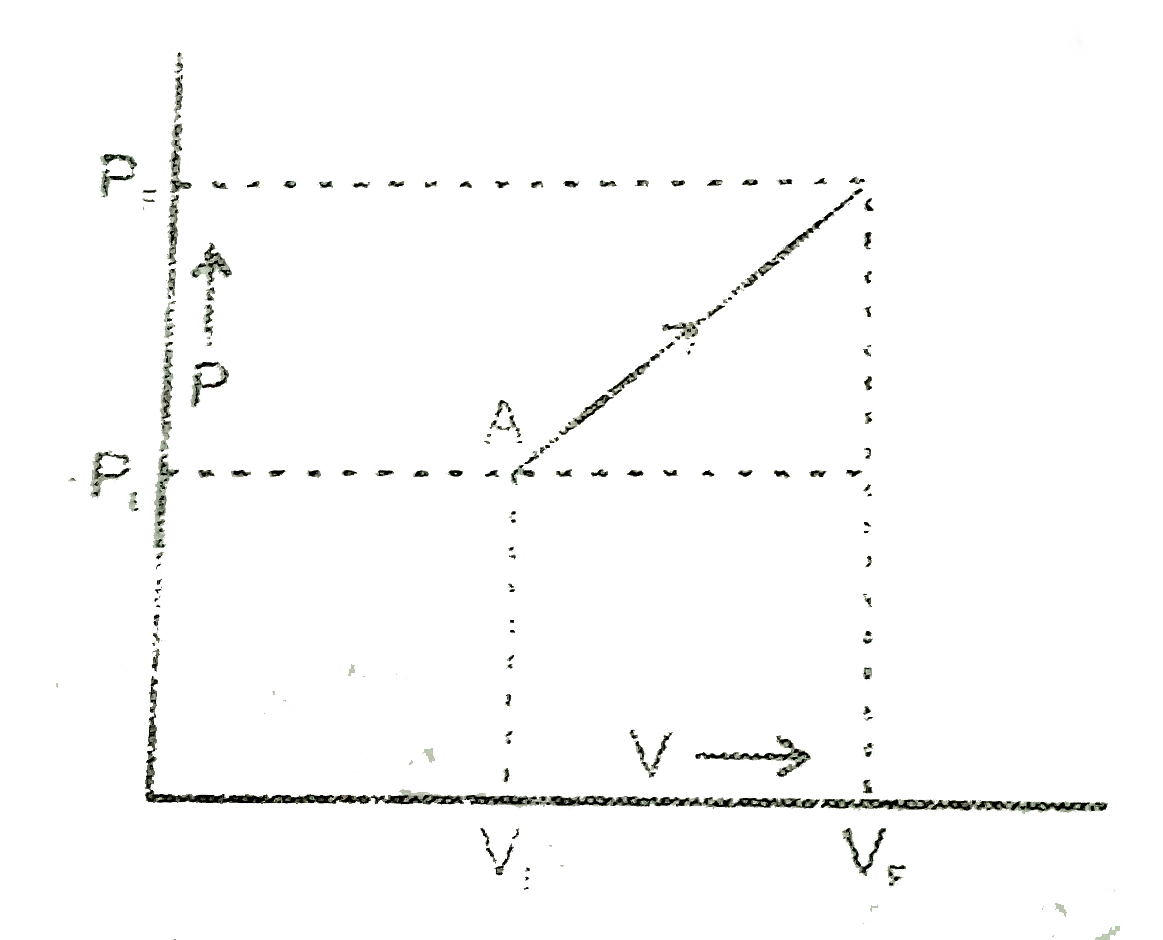
Text Solution
Verified by Experts
Topper's Solved these Questions
KTG & THERMODYNAMICS
RESONANCE|Exercise Board Level Exercise|1 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise SECTION (B)|3 VideosKTG & THERMODYNAMICS
RESONANCE|Exercise Solved problem|1 VideosKINETIC THEORY OF GASES AND THERMODYNAMICS
RESONANCE|Exercise Exercise|64 VideosMAGNETIC FIELD AND FORCES
RESONANCE|Exercise Exercise|65 Videos
Similar Questions
Explore conceptually related problems