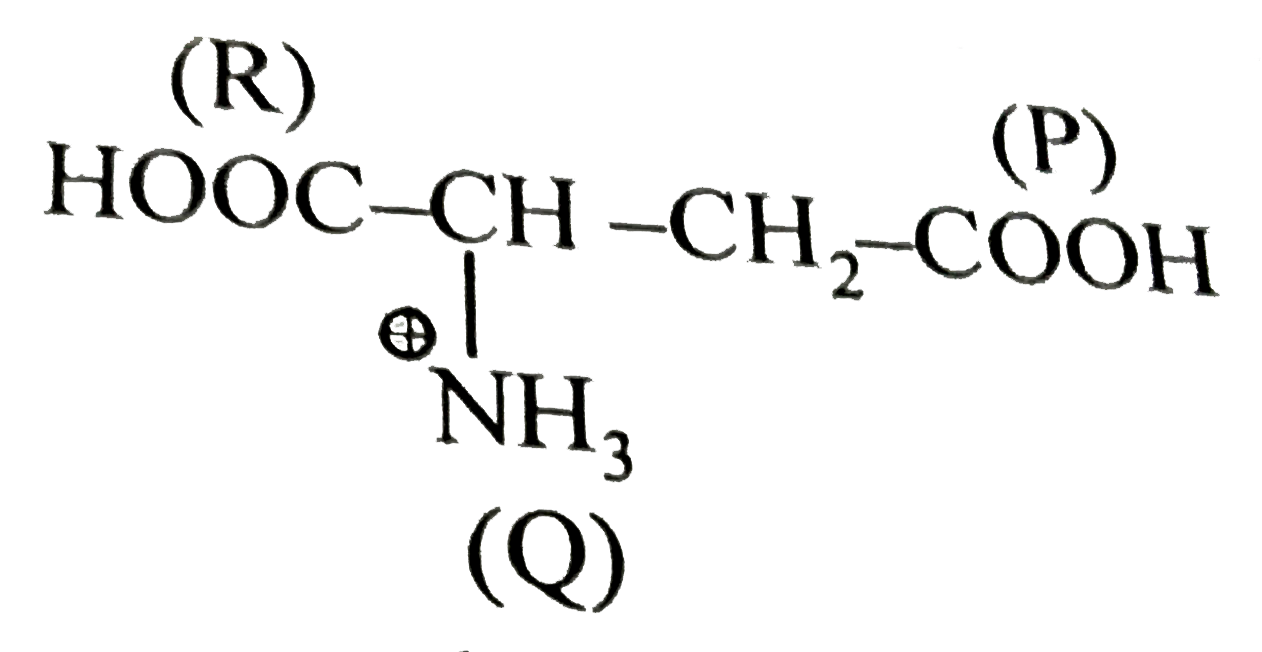Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-GENERAL ORGANIC CHEMISTRY-Exercise 3
- The pKa values for the three acidic group P,Q,R are 4.3, 9.7 and 2.2 r...
Text Solution
|
- Number of 1^(@) and 2^(@) alcoholic group ini sucrose and x and y valu...
Text Solution
|
- Number of initial centre in maltose are.
Text Solution
|
- Number of chiral carbons in Frischer projection of D-Fructose in which...
Text Solution
|
- Number of chiral carbon in Haworth structure of alpha-Glucopyranose ha...
Text Solution
|
- Saccharic acid can be obtained by oxidation of glucose number of C, H ...
Text Solution
|
- D-Glucose, D-Mannose and D-Fructose form same osazone on reaction with...
Text Solution
|
- Give the structures of the products in each of the following reaction....
Text Solution
|
- Aspartame, an artificial sweetener, is a peptide and has the following...
Text Solution
|