Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-COORDINATION COMPOUNDS-SBJECTIVE TYPE
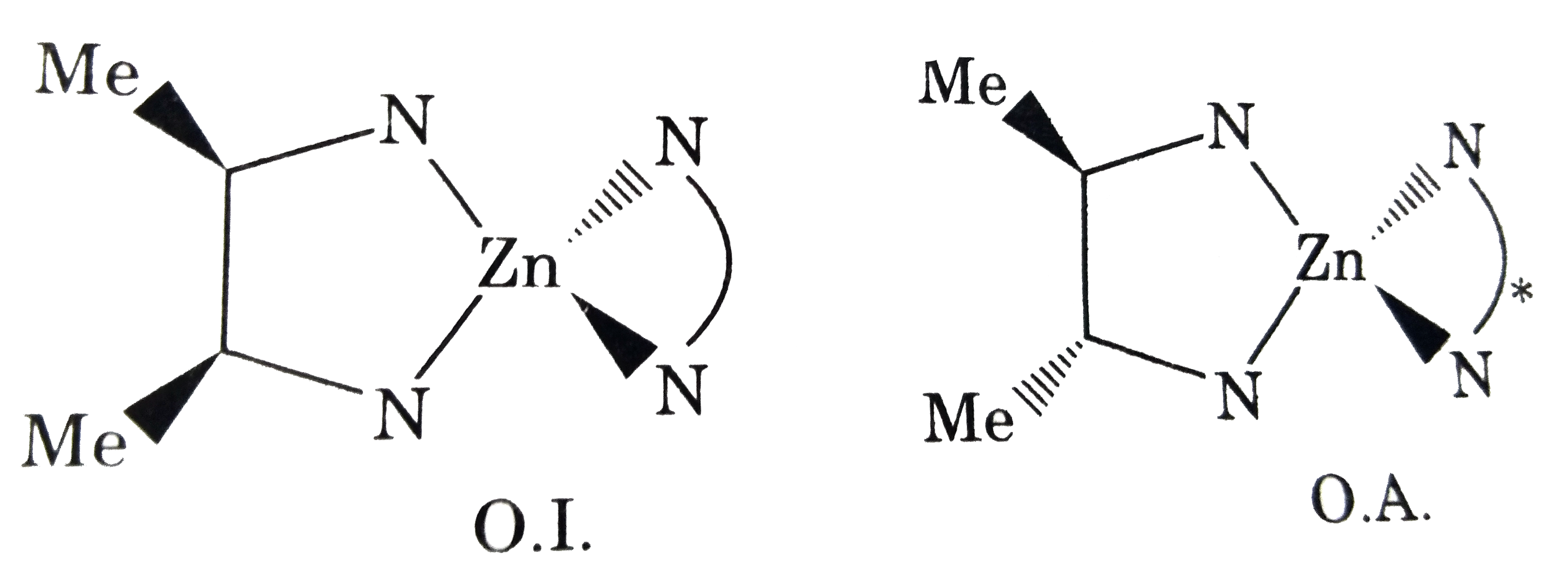
- Find the total number of optically active compounds in [Zn(bn)(en)]Cl(...
Text Solution
|
- Find the total number of ligands which act as pi-acceptor. O^(2), F^...
Text Solution
|
- How many of the following species would have C-O bond length longer th...
Text Solution
|
- How many of the modern formula are correctly matched with the structur...
Text Solution
|
- Find the sum of optically active isomers of both [Pt(gly)(2)Cl(2)]" an...
Text Solution
|
- Find the total number of diamagnetic complexes. K(3)[Fe(CN)(6)],[Co(...
Text Solution
|
- Consider the following complex entities. [FeF(6)]^(3-), [Fe(H(2)O)(6...
Text Solution
|
- Which of the following is tetrahedral and paramagnetic ? MnO(4)^(-),...
Text Solution
|
- Find the number of paramagnetic species each central metal ion having ...
Text Solution
|
- Which of the following compounds are coloured due to charge transfer ?...
Text Solution
|
- How many compounds are tetrahedral in shape ? (a) KMnO(4)" "(b) K(...
Text Solution
|
- [(H(2)O)(8)Fe(2)(OH)(2)].(SO(4))(2) Calculate the coordination numbe...
Text Solution
|
- Find the number of compound which has four orbitals participating in h...
Text Solution
|
- How many of the following has 2.8 B.M. magnetic moment ? (a) [Ni(NH(...
Text Solution
|
- Calculate the coordination number and number of stereo isomers of [C...
Text Solution
|
- Find the value of m+n in the anionic species [Ti(CO)mp^(n-), if it fol...
Text Solution
|
- Find the number of species having C-O bond length grater than that in ...
Text Solution
|
- Consider the complex [Co(NH(3))(4)CO(3)]ClO(4), in which coordination ...
Text Solution
|
- Select the total number of properties which are correct with respect t...
Text Solution
|
- How many of the following obey's Sidgwick EAN rule ? (a) K(4)[Fe(CN)...
Text Solution
|