A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- It is not possible to explain the Pauli's exclusion principal with th...
Text Solution
|
- The subshell that rises after f subshell is called g subshell What i...
Text Solution
|
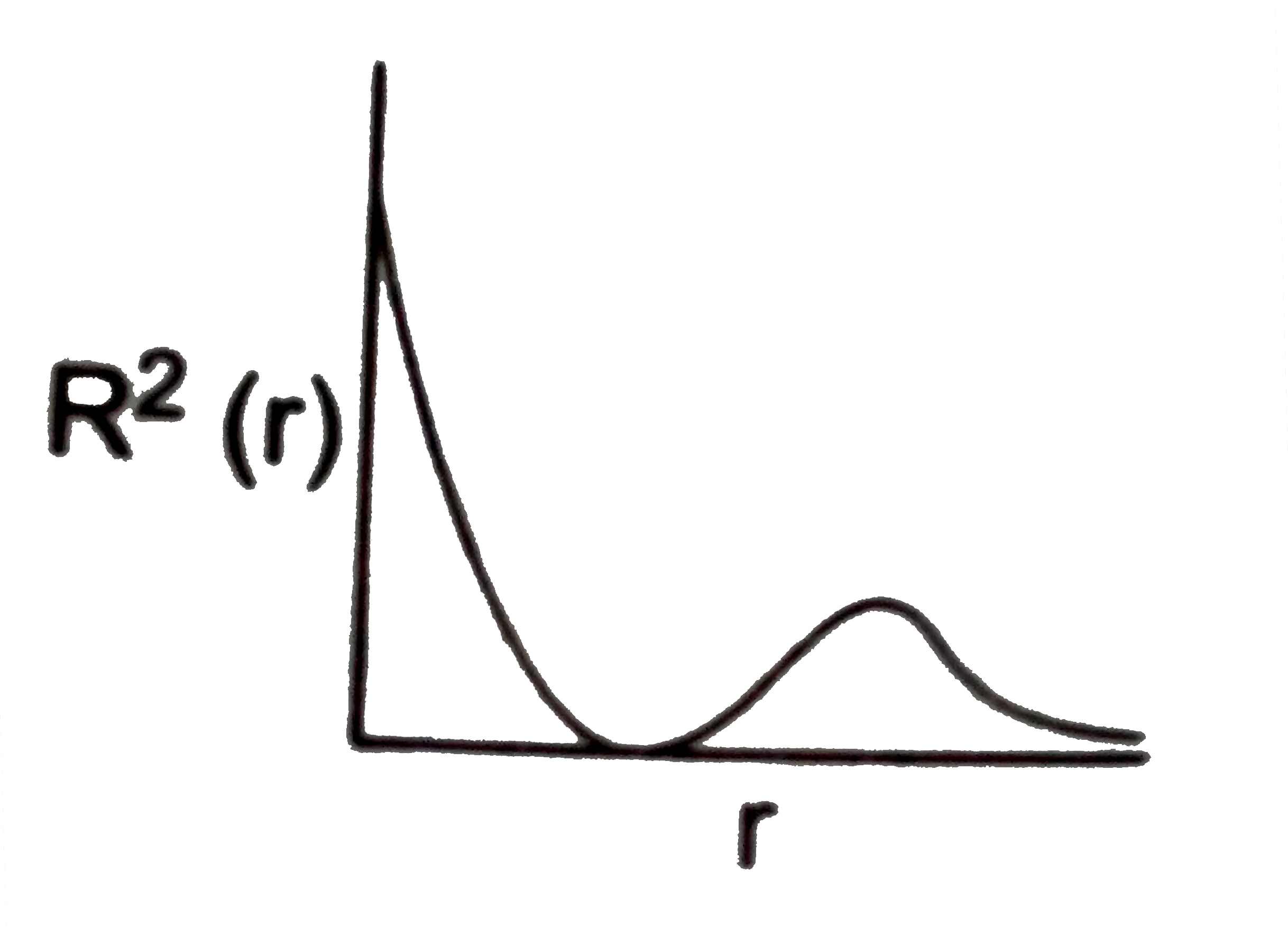
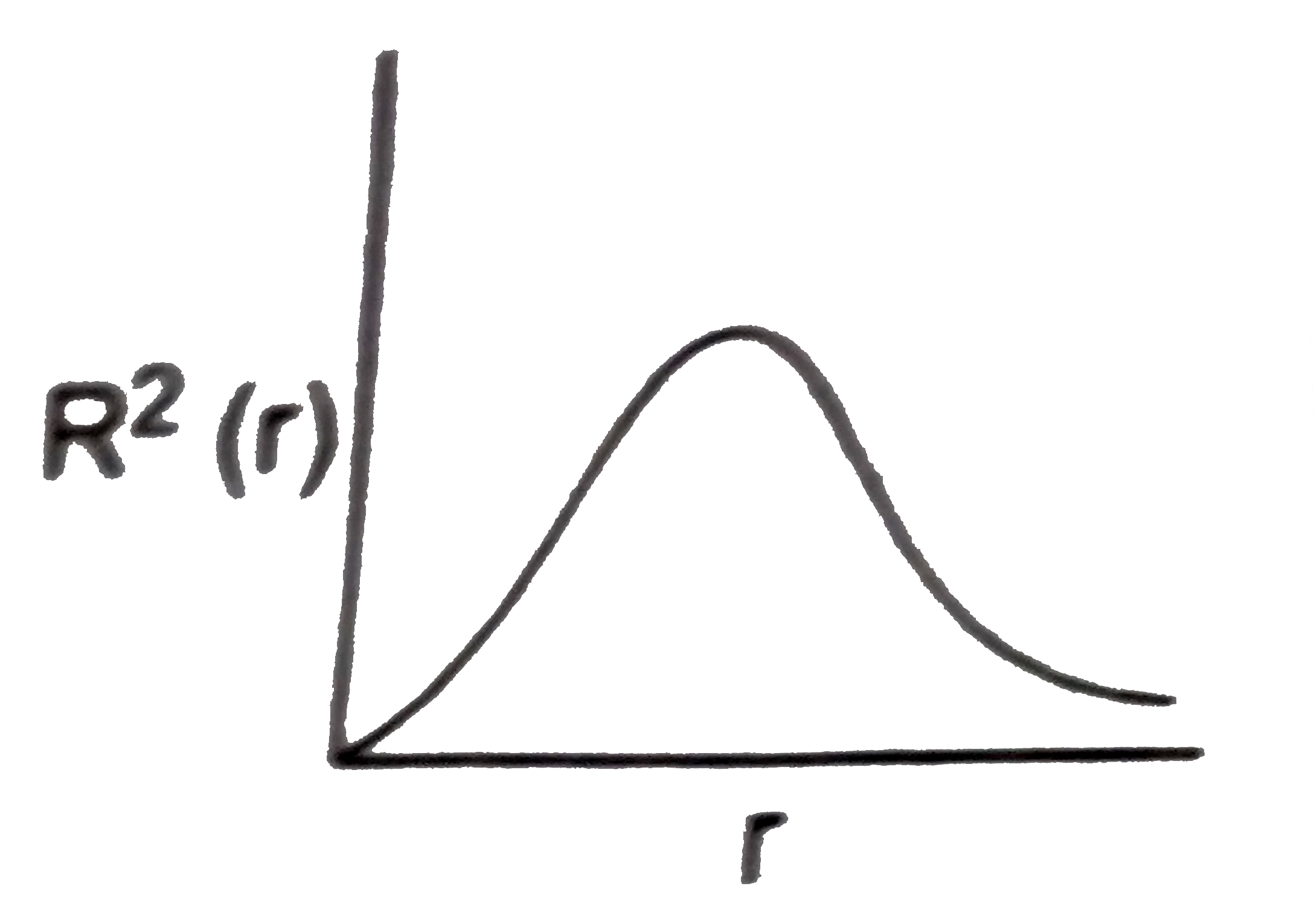
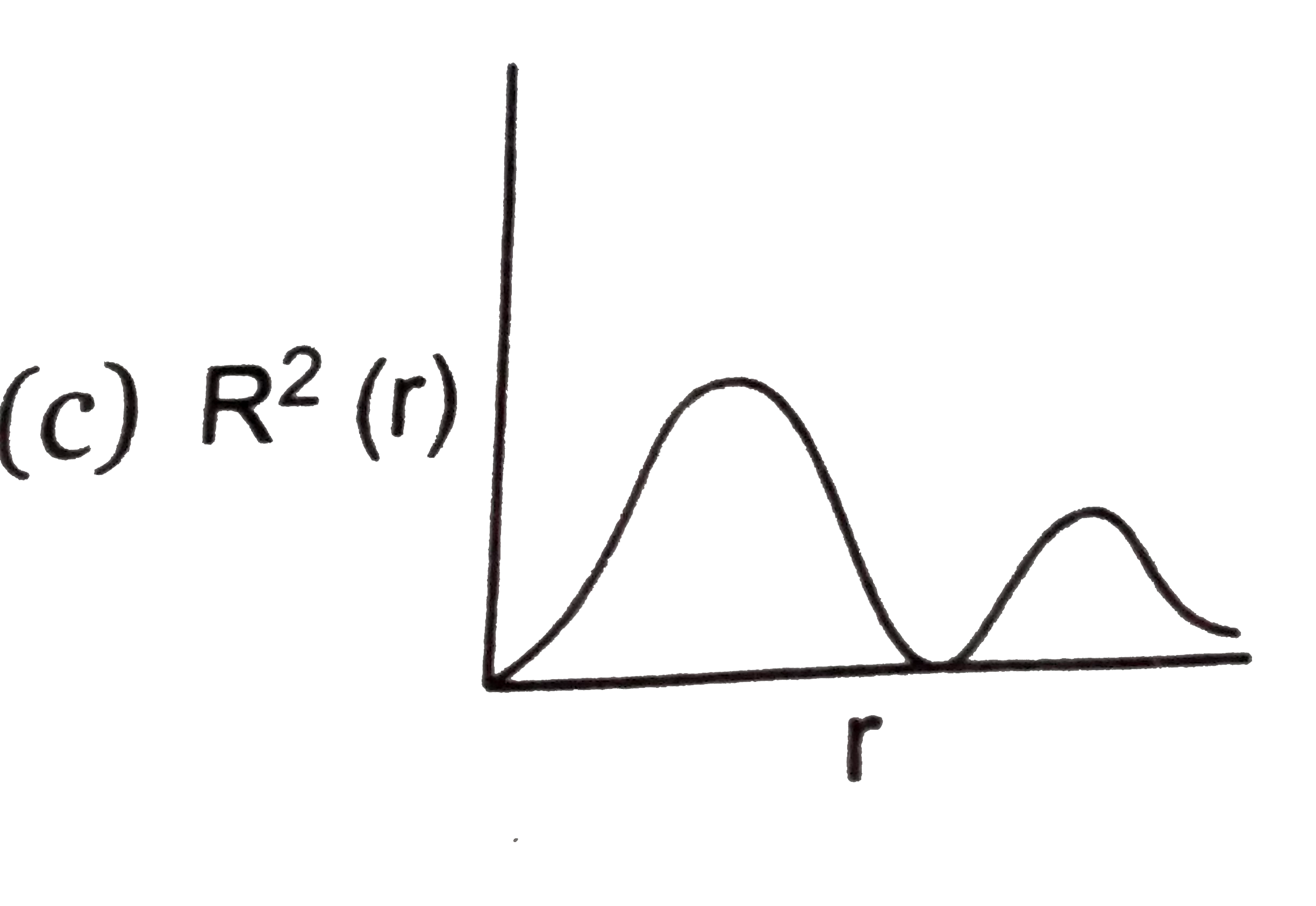
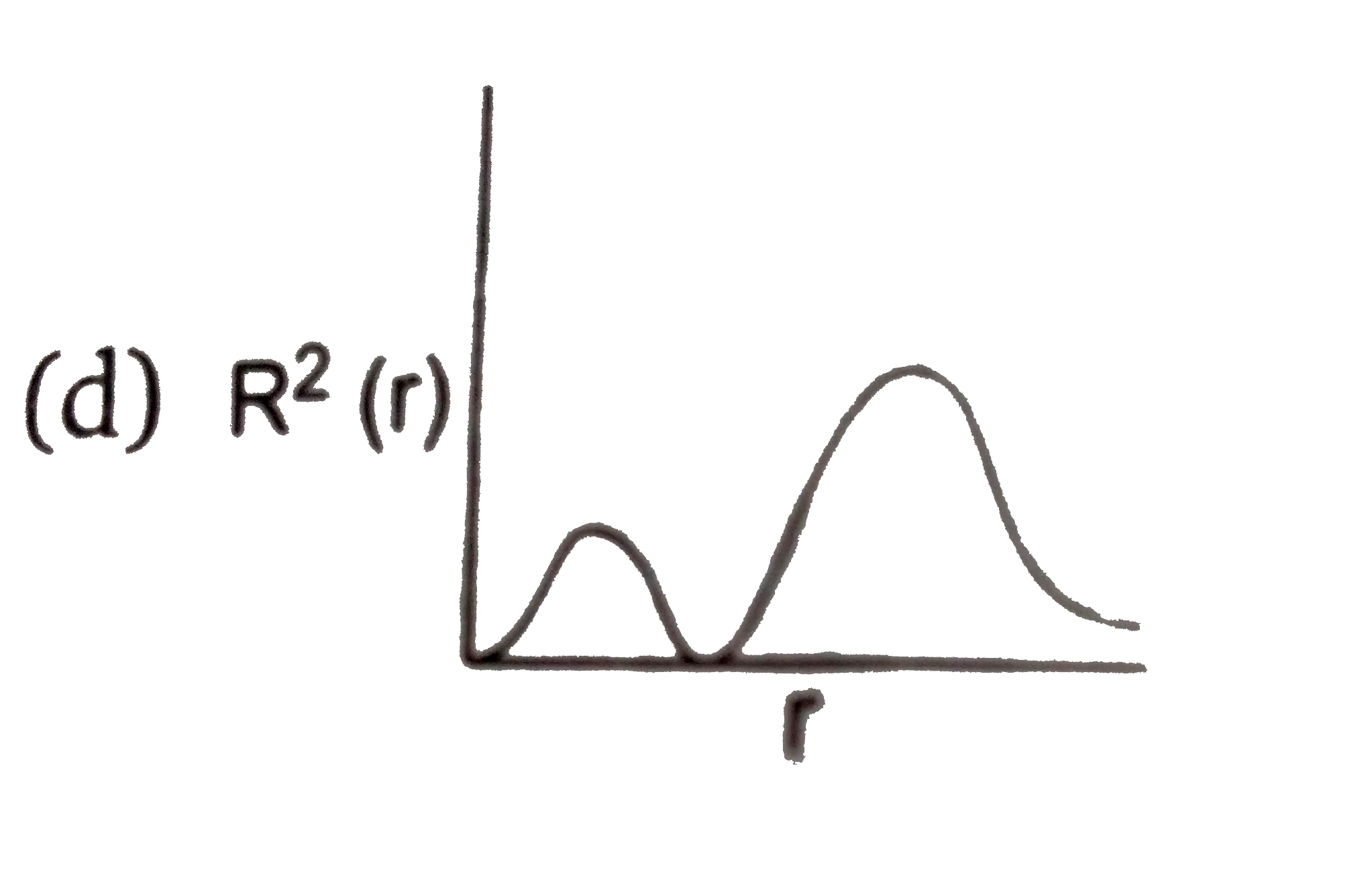
- The variation of radial probability density R^2 (r) as a function of ...
Text Solution
|
- In iron atom, how many electrons have n=3 and l=2?
Text Solution
|
- If n and l are respectively the principal and azimuthal quantum number...
Text Solution
|
- Maximum number of nodes are present in :
Text Solution
|
- The possible correct set of quantum numbers for the unpaired electron ...
Text Solution
|
- The aufvau principle implies that a new electron will center an orbita...
Text Solution
|
- the orbital diagram in which aufbau principal is violated is :
Text Solution
|
- Consider the following six electronic configurations (remaining inner ...
Text Solution
|
- Which of the following set of quantum numbers shows orbital of highest...
Text Solution
|
- A subshell n = 5, l = 3 can accommodate :
Text Solution
|
- In H-atom energy of electron is datermined by :
Text Solution
|
- In iron atom, how many electrons have n=3 and l=2?
Text Solution
|
- How many electrons in an atom can have n = 4, l =2 ,m= -2 and s = +1/...
Text Solution
|
- The degencracy of 1st excited state of H atom is (Ignore efffect of s...
Text Solution
|
- Not considering the electron spin, the degeneracy of second excited st...
Text Solution
|
- Which orbital has only positive value of wave function at all distance...
Text Solution
|
- Four electrons in aan atom have the set of quantum numbers as given be...
Text Solution
|
- The set of quantum numbers, n = 3, l = 2, m(l) = 0
Text Solution
|