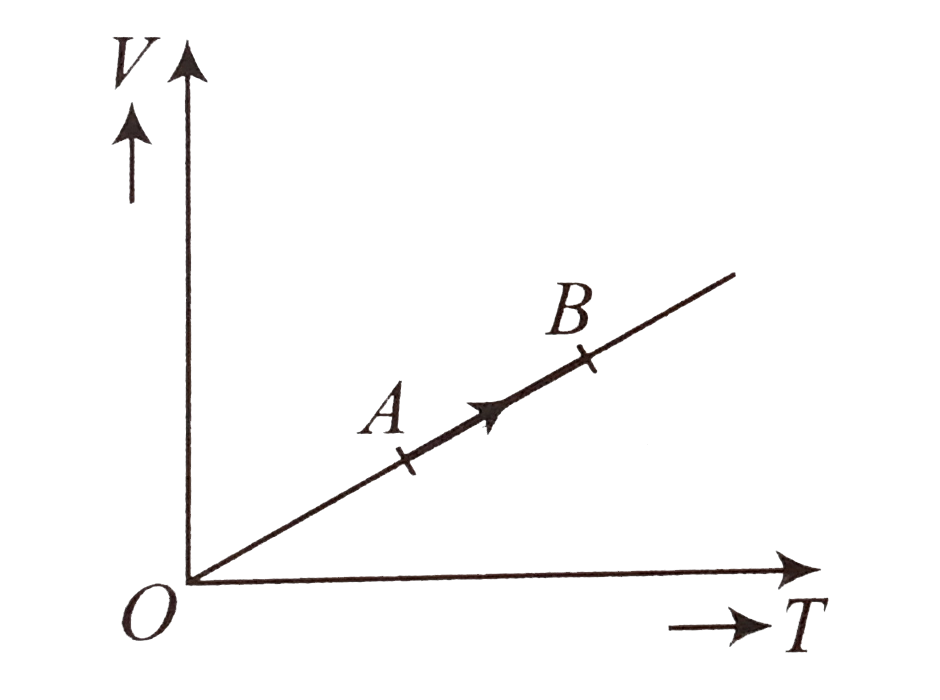
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY|Exercise Solved paper 2018(AIIMS)|28 VideosSOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY|Exercise Solved paper 2018(JIPMER)|38 VideosSOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY|Exercise Solved Papers 2017(JIPMER)|28 VideosSIMPLE HARMONIC MOTION
DC PANDEY|Exercise Integer type questions|14 VideosSOUND WAVES
DC PANDEY|Exercise Exercise 19.7|4 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-SOLVD PAPERS 2017 NEET, AIIMS & JIPMER-Solved paper 2018(NEET)
- The volume (V) of a monatomic gas varies with its temperature (T) as s...
Text Solution
|
- The fundamental frequency in an open organ pipe is equal to the third ...
Text Solution
|
- At what temperature , will the rms speed of oxygen molecules be suffic...
Text Solution
|
- The efficiency of an ideal heat engine working between the freezing po...
Text Solution
|
- A tuning fork is used to produce resonance in glass tube. The length o...
Text Solution
|
- A pendulum is hung the roof of a sufficiently high building and is mov...
Text Solution
|
- A body initially rest and sliding along a frictionless trick from a he...
Text Solution
|
- There object, A : (a solid sphere), B : (a thin circular disk) and C :...
Text Solution
|
- A moving block having mass m , collides with another stationary block ...
Text Solution
|
- Which one of the following statements is incorrect?
Text Solution
|
- A toy car with charge q moves on a frictionless horizontal plane surfa...
Text Solution
|
- A block of mass m is placed on a smooth inclined wedge ABC of inclinat...
Text Solution
|
- The moment of the force, vec F = 4 hati + 5 hat j - 6 hat k at (2, 0 ,...
Text Solution
|
- A student measued the diameter of a small steel ball using a screw gau...
Text Solution
|
- A solid sphere is rotating in free space. If the radius of the sphere...
Text Solution
|
- The kinetic energies of a planet in an elliptical orbit about the Sun,...
Text Solution
|
- If the mass of the sun were ten times smaller and the universal gravit...
Text Solution
|
- A solid sphere is in rolling motion. In rolling motion a body prossese...
Text Solution
|
- A small sphere falls from rest in a viscous liquid. Due to frication, ...
Text Solution
|
- The power radiated by a black body is P, and it radiates maximum energ...
Text Solution
|