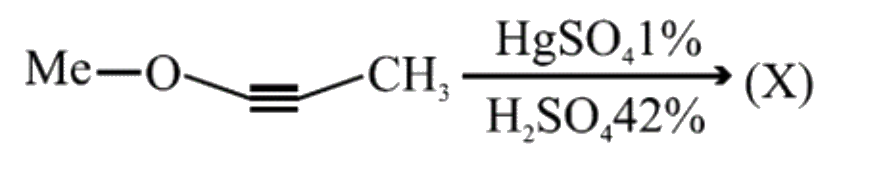
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
NTA MOCK TESTS-NEET MOCK TEST 20-CHEMISTRY
- Complete the following reaction
Text Solution
|
- The pH of 0.5 M aqueous solution of HF (K(a)=2xx10^(-4)) is
Text Solution
|
- A negatively charged sol can be formed by peptizing a solution of
Text Solution
|
- Which of the following compound is most rapidly hydrolysed by S(N)1 me...
Text Solution
|
- The destruction of the biological bature and activity of proteins by h...
Text Solution
|
- Which of the following cations is detected by the flame test?
Text Solution
|
- In the oxymercuration - demercuration of the following compound H(2)...
Text Solution
|
- There is no d-d transition in Cu^(+) but Cu(2)O is coloured due to
Text Solution
|
- The degree of hydrolysis of which of the following salt is independent...
Text Solution
|
- CH(3)CH=CHCHO is oxidised to CH(3)-CH=CHCOOH, using oxidising agent as...
Text Solution
|
- Likely bond angles of SF(4) molecule are :
Text Solution
|
- k(2)CO(3) cannot be prepared by solvay process because
Text Solution
|
- Metal M+"air" overset(delta)(to)Aoverset(H(2)O)(to)Boverset(HCl)(to) W...
Text Solution
|
- Equal weight of CH(4) " and " H(2) are mixed in an empty container at ...
Text Solution
|
Text Solution
|
- The correct statement is
Text Solution
|
- The bond dissociation energies for Cl(2), I(2) and IC l are 242.3, 151...
Text Solution
|
- The bond length the species O(2), O(2)^(+) and O(2)^(-) are in the ord...
Text Solution
|
- Which reaction gives colloidal solution
Text Solution
|
- How much will the reduction potential of a hydrogen electrode change w...
Text Solution
|