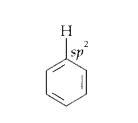Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUES-EXERCISE
- Given the hybridistaiton state of each carbon in the following compoun...
Text Solution
|
- Indicate the sigma- and pi - bonds in the following molecules: I. C(...
Text Solution
|
- Write the bond line formula for the following compounds: I. Isopropy...
Text Solution
|
- Give the IUPAC names of the following compounds :
Text Solution
|
- Which of the following represents the corrcet IUPAC name for the compo...
Text Solution
|
- Draw the formulae for the first five numbers of each homologous series...
Text Solution
|
- Give condensed and bond line structural formulas and identify the func...
Text Solution
|
- Identify the functional groups in the following compounds
Text Solution
|
- Which of the two: O(2)NCH(2)CH(2)O^(–) or CH(3)CH(2)O^(–) is expecte...
Text Solution
|
- Explain why alkyl groups act as electron donors when attacted to a pi-...
Text Solution
|
- Draw the resonance structures for the following compounds. Show the el...
Text Solution
|
- What are electrophiles and nucleophiles ? Explain with examples.
Text Solution
|
- Identify the reagents shown in bold in the following equations as nucl...
Text Solution
|
- Classify the following reactions in one of the reaction type studied i...
Text Solution
|
- What is the relationship between the members of the following pairs of...
Text Solution
|
- For the following bond cleavages, use curved-arrow to show the electro...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Give a brief description of the principles of the following techniques...
Text Solution
|
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|