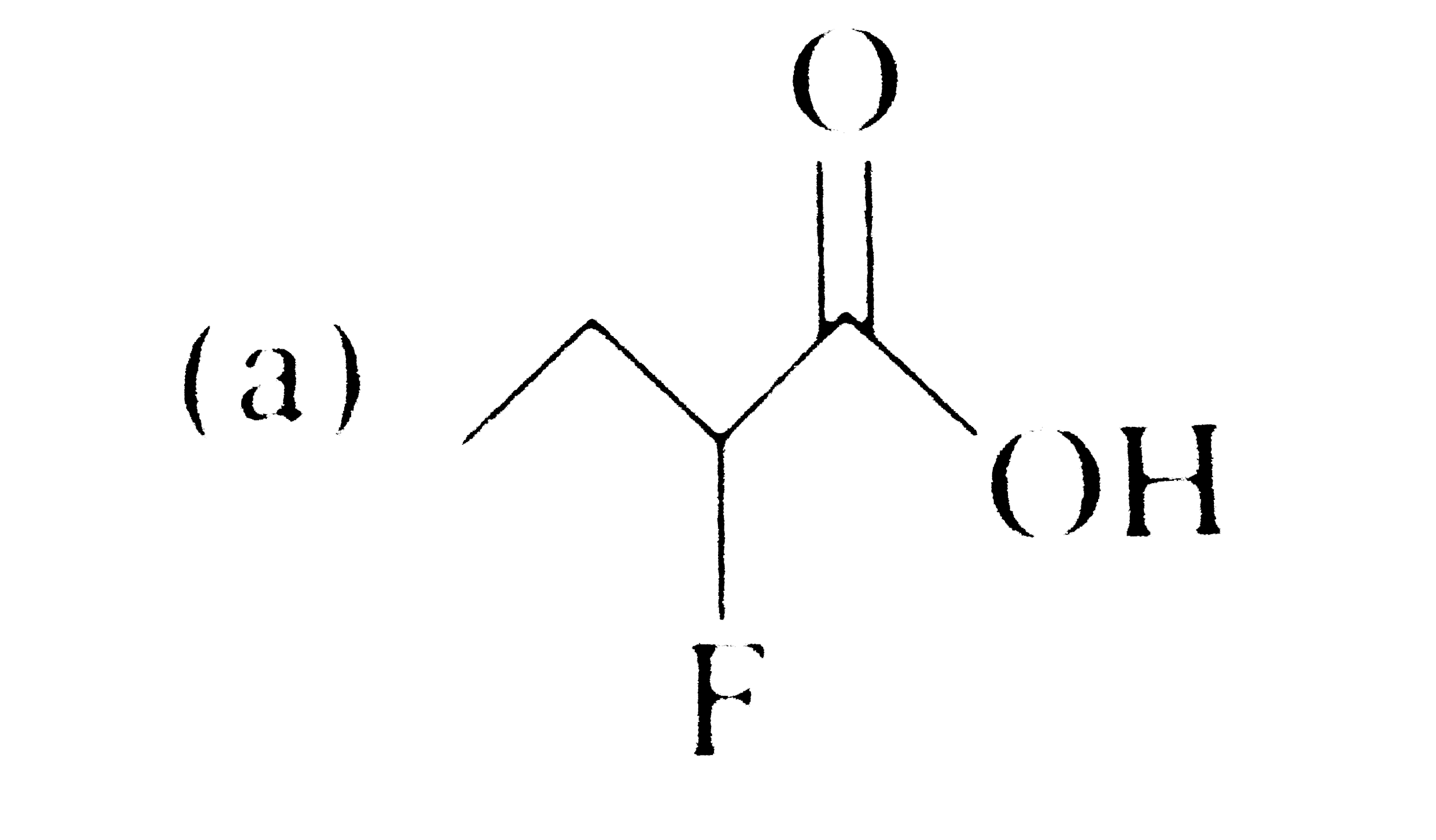
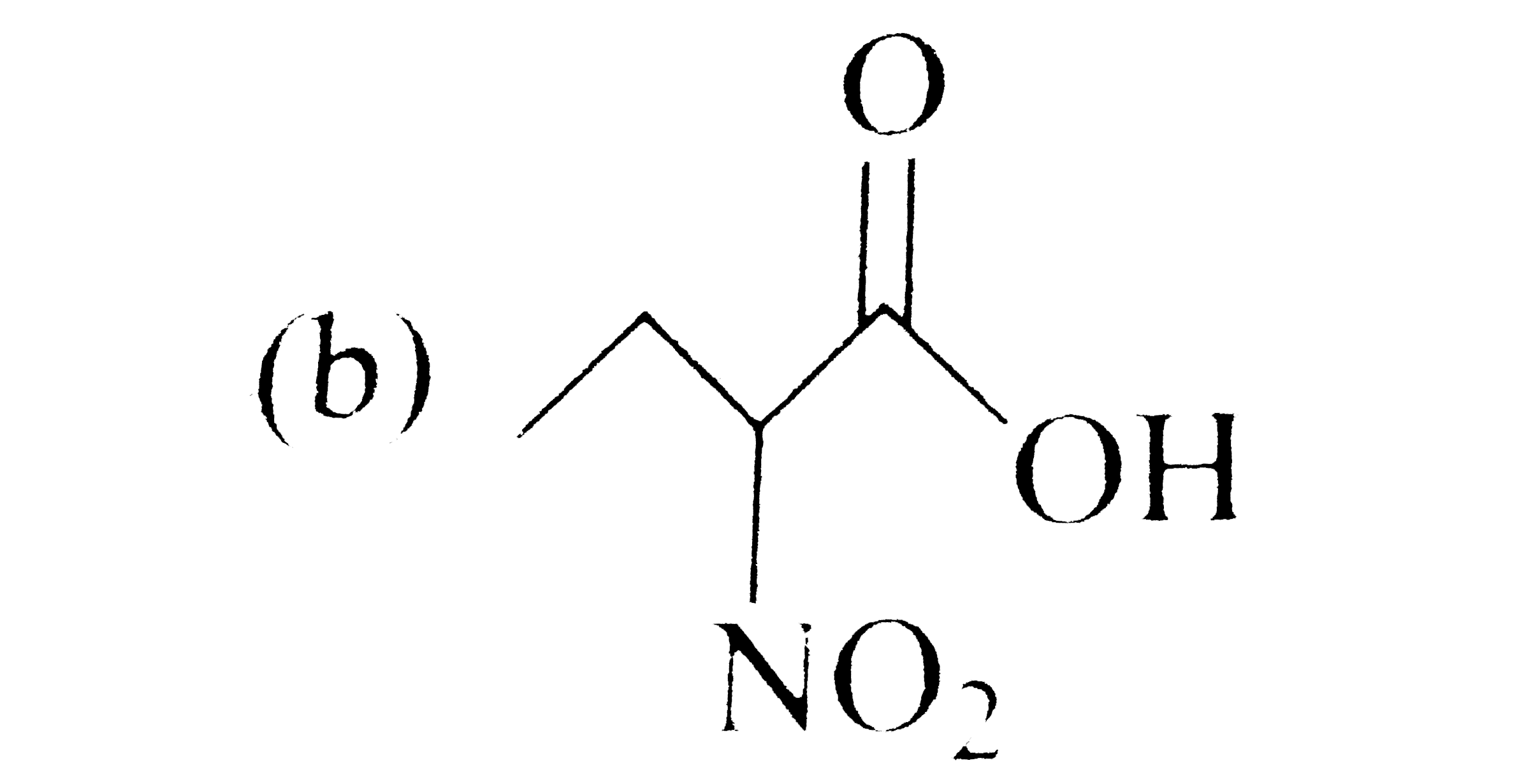
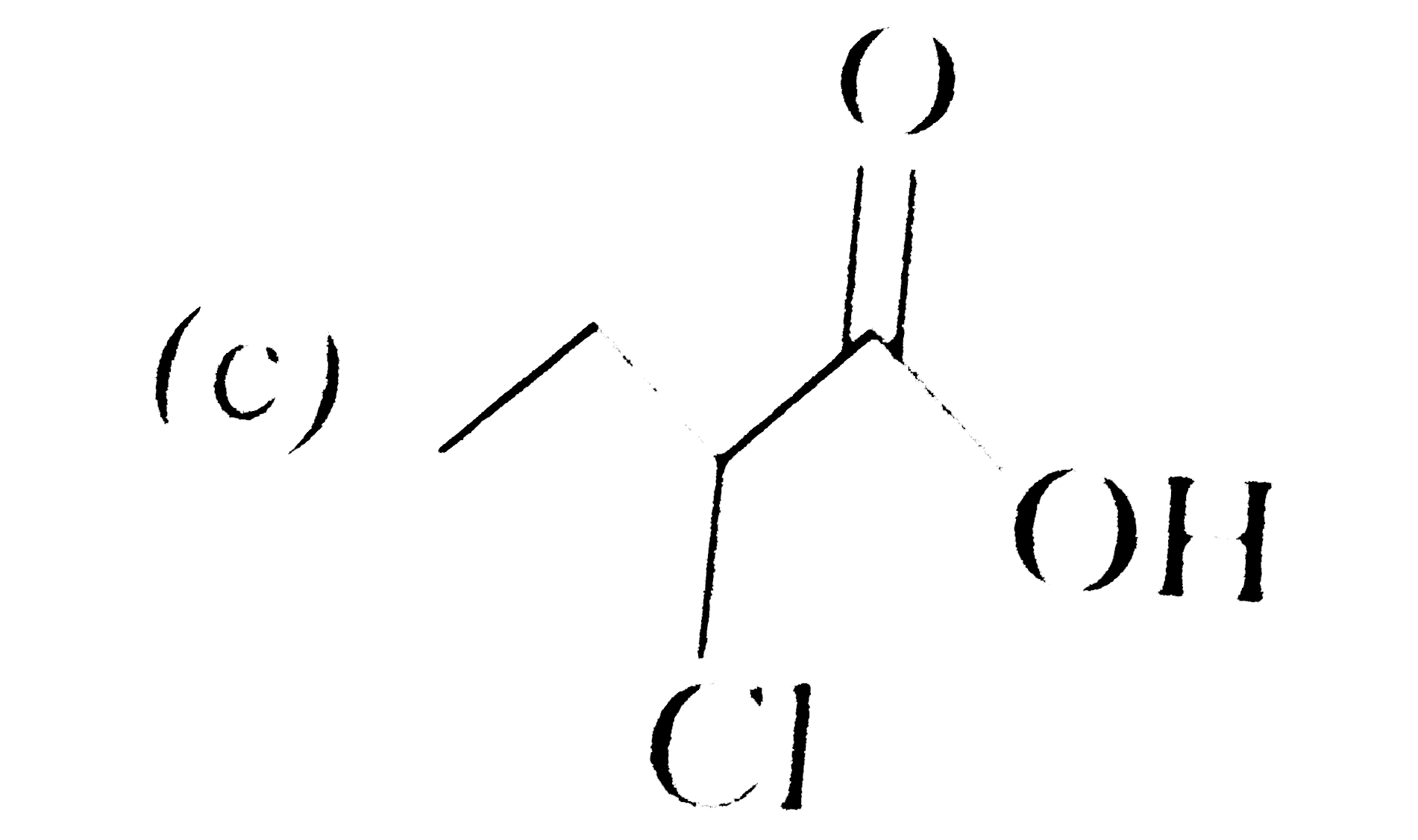
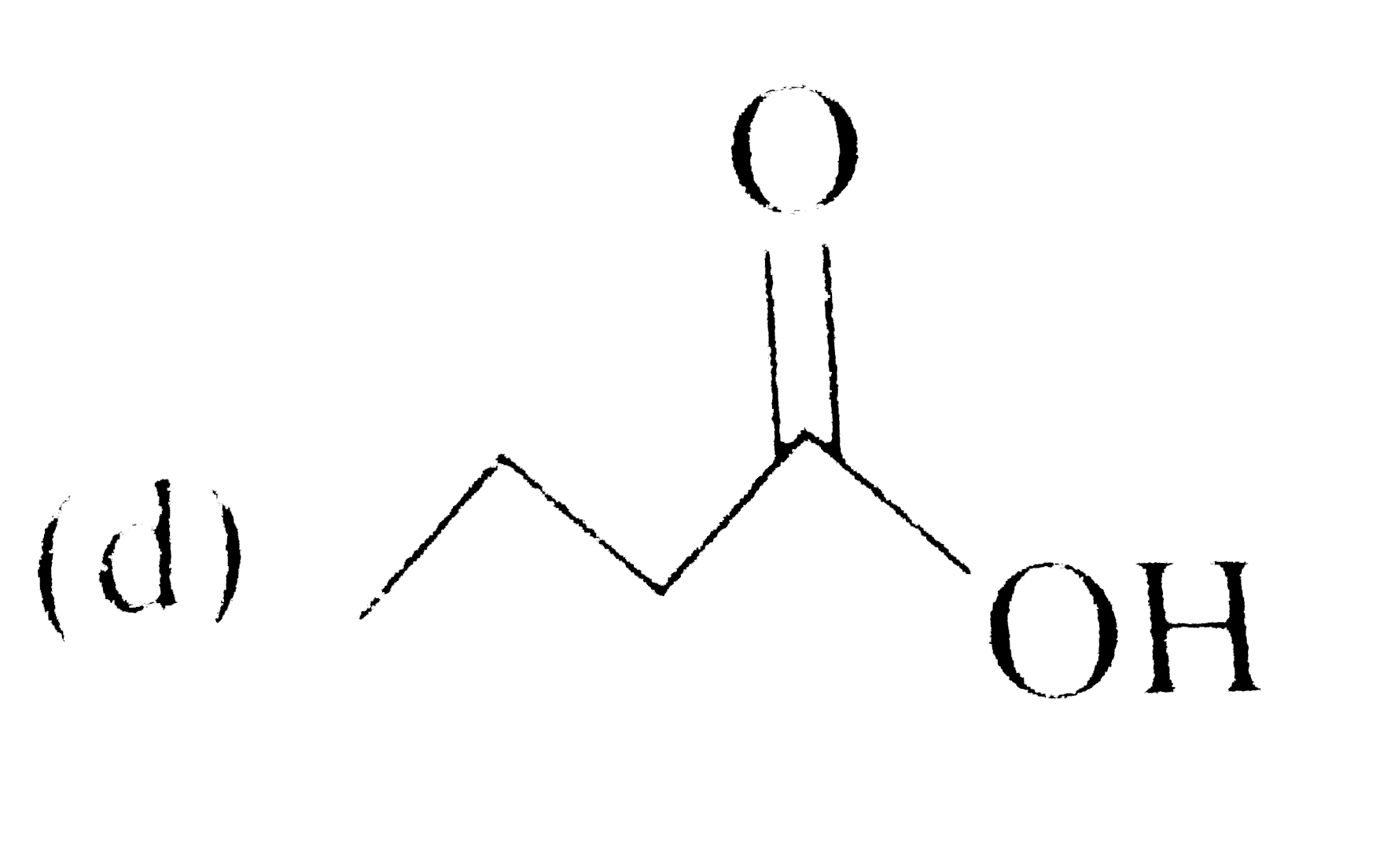
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Match The Column|9 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|10 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.1 To Q.25)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems