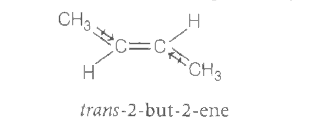A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2019 (3 MAY SHIFT 2)-CHEMISTRY
- Match the following : The correct answer is
Text Solution
|
- Find the suitable IUPAC name of the compound given below underset(OH...
Text Solution
|
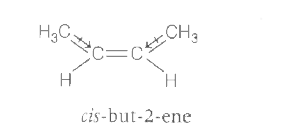
- The boiling poing (in K) of cis but -2- ene and dipole moment ( in D) ...
Text Solution
|
- The major product formed in the following reaction sequence is
Text Solution
|
- NaCl is fcc lattice , where Na^+ ions are at corner and face centre po...
Text Solution
|
- How many grams of glucose are required to prepare an aqueous solution ...
Text Solution
|
- If 0.1 M solution of NaCl is isotonic with 1.1 w % urea solution, the ...
Text Solution
|
- An electrolyte of a polymer -salt complex of poly (ethylene oxide ) L...
Text Solution
|
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- The mass of haemoglobin in mg required to protect from coagulation of ...
Text Solution
|
- In the preparation of chloration of chlorine by the electrolysis of br...
Text Solution
|
- Which is the correct equation for the reaction of AgCl with NH4OH ?
Text Solution
|
- Name the gaseous products from the following A and B reactions respect...
Text Solution
|
- The elements with the highest and lowest enthalpy of atomisation , res...
Text Solution
|
- The IUPAC name of the compound (NH4)2[Ni (C2O4)2(H2O)2] is
Text Solution
|
- Which one of the following is a biodegradable polymer ?
Text Solution
|
- The enzyme responsible for the conversion of proteins to alpha - amin...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- Which one of the following is used to obtain the maximum percentage of...
Text Solution
|
- Find the correct order of acid strengths of the following compounds :
Text Solution
|