Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise VALUE BASED QUESTIONS|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise PROMBEMS FOR PRACTICE|38 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DINESH PUBLICATION|Exercise ADDITIONAL IMPORTANT QUASTIONS|41 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
DINESH PUBLICATION|Exercise Unit test|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
DINESH PUBLICATION|Exercise All Questions|310 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -H.O.T.S. CONCEPTUAL QUESTIONS
- Atom A,B and C occur in the same period and have one, six and seven va...
Text Solution
|
- Interpret non-linear shape of H(2)S and non-planar shape of PCl(3) on ...
Text Solution
|
- In a polar solvent, PCl(5) undergoes an ionization reaction as : " ...
Text Solution
|
- In the equation, A+2B+H(2)O to C+2D(A=HNO(2),B=H(2)SO(3),C=NH(2)OH) id...
Text Solution
|
- In which of the following pairs, the two species are iso-structural ? ...
Text Solution
|
- Answer the following : (a) C Cl(4) is non-polar but CH(3)Cl is polar...
Text Solution
|
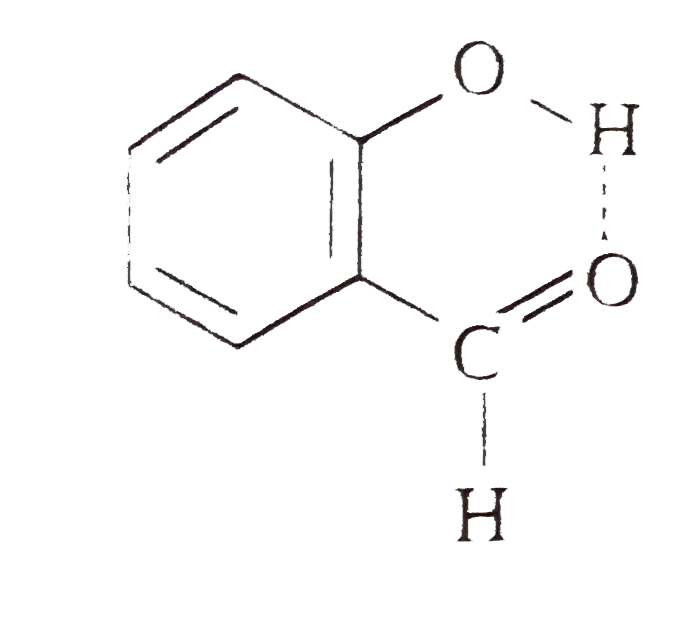
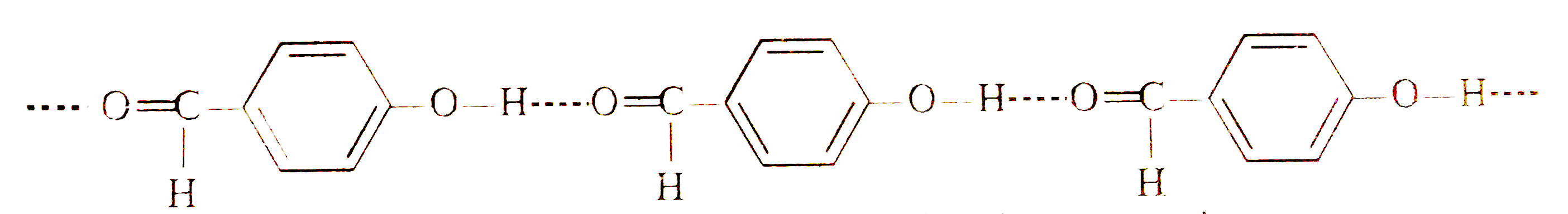
- Explain why is o-hydroxybenzaldehyde a liquis at room temperaturre whi...
Text Solution
|
- (a) H(2)^(+)and H(2)^(-) ions have same bond order but H(2)^(-) ion is...
Text Solution
|
- State with reasons : (i) Which is more acidic : anhydrous HCl or aqu...
Text Solution
|
- Assign reason for the following : (i) Ammonis is soluble in water wh...
Text Solution
|
- Drw all the possible resonating structures for azide ion (N(3)^(-)ion)...
Text Solution
|