Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Practice Test|7 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Some Typical Conversions|30 VideosALDEHYDES AND KETONES
DINESH PUBLICATION|Exercise Question From Board|86 VideosALCOHOLS AND PHENOLS
DINESH PUBLICATION|Exercise Matrix|9 VideosALKALI EARTH METALS
DINESH PUBLICATION|Exercise Unit test-12|5 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALDEHYDES AND KETONES -Higher Order Thinking Skills
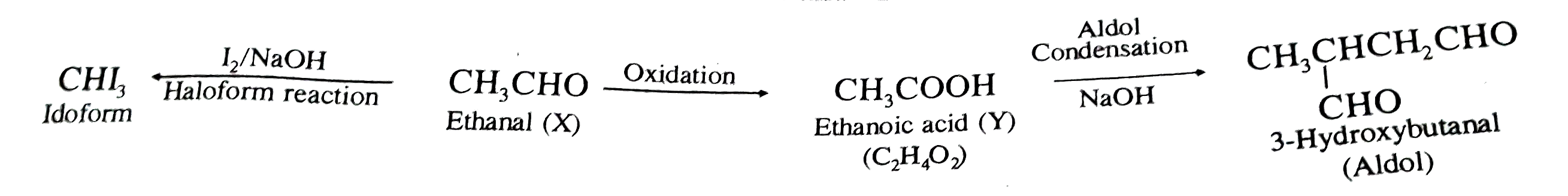
- A compound 'X' (C(2)H(4)O) on oxidation gives 'Y' (C(2)H(4)O(2)). The ...
Text Solution
|
- One mole of symmetrical alkene upon ozonolysis gives two moles of an a...
Text Solution
|
- Out of the following compounds, which will be dehydrated most readily?
Text Solution
|
- Give the structure of the compounds in the following reactions CH(2)...
Text Solution
|
- Complete the following
Text Solution
|
- How will you bring about the following conversions ?
Text Solution
|