A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 94-CHEMISTRY
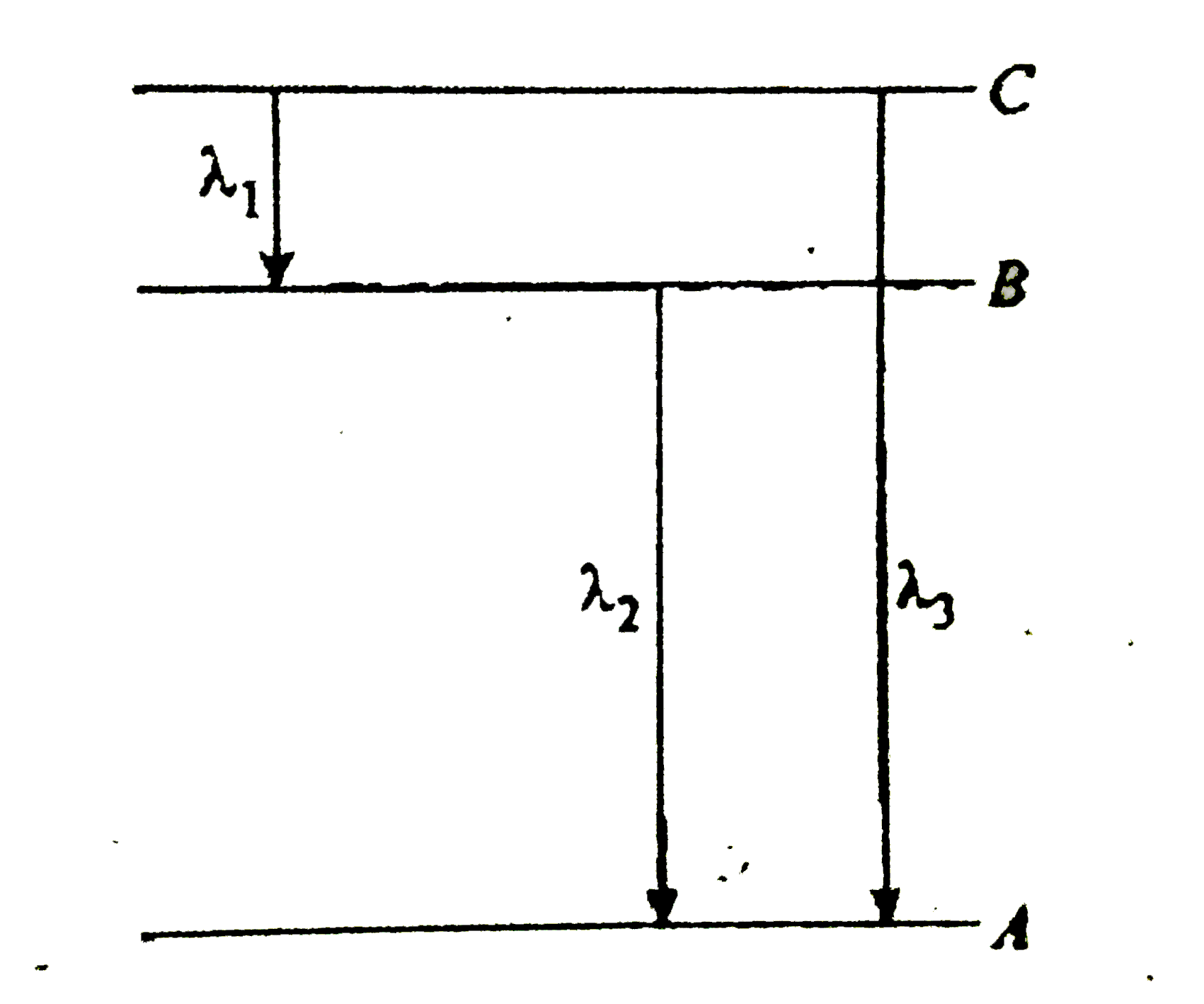
- Energy levels A,B and C of a certain atom correspond to increasing val...
Text Solution
|
- 0.15 mole of pyridinium chloride has been added into 500 cm^(3) of 0.2...
Text Solution
|
- Which among the following compound has [L] configuration
Text Solution
|
- The temperature coefficient of a cell whose operation is based on the ...
Text Solution
|
- Which of the following Lewis dot structure of CO2 is incorrect ?
Text Solution
|
- How many sigma and pi bonds are present in methyl acrylate
Text Solution
|
- If the radii of A^+ and B^- in the crystalline solid AB are 96 pm and ...
Text Solution
|
- Consider the following reaction In the above reaction reagent / c...
Text Solution
|
- An ideal gas expands from 10^(-3) m^(3) to 10^(-2) m^(3) at 300 K agai...
Text Solution
|
- Which one of the following arrangements of molecules is correct on the...
Text Solution
|
- The conductivity of 0.001 M acetic acid is 5xx10^(-5)S cm^(-1) and ^^^...
Text Solution
|
- In which reaction product formation takes place by Saytzeff rule
Text Solution
|
- The coagulation of 100ml of colloidal solution of gold is completely p...
Text Solution
|
- The magnetic moment of complex given below are in the order: (I) [Ni...
Text Solution
|
- Number of moles of K(2)Cr(2)O(7) can be reduced by 1 mole of Sn^(2+) i...
Text Solution
|
- In given reaction [X] and [Y] respectively are CH3-overset(O)overset(|...
Text Solution
|
- Fe(2)O(2)(s)+(3)/(2)C(s)to(3)/(2)CO(2)(g)+2Fe(s) DeltaH^(@)=+234.12K...
Text Solution
|
- Arrange decreasing order of reactivity of these compounds for nucleoph...
Text Solution
|
- In an electroplating experiment m g of silver is deposited, whe 4 ampe...
Text Solution
|
- The complexes [Co(NH3)4(H2O)Cl] Br2 and [Co(NH3)4Br2]Cl.H2O are exampl...
Text Solution
|