Text Solution
Verified by Experts
Topper's Solved these Questions
SAMPLE QUESTION PAPER (CHEMISTRY )
CBSE MODEL PAPER|Exercise SECTION C|11 VideosSAMPLE QUESTION PAPER (CHEMISTRY )
CBSE MODEL PAPER|Exercise SECTION D|1 VideosSAMPLE QUESTION PAPER (CHEMISTRY )
CBSE MODEL PAPER|Exercise SECTION A (OBJECTIVE TYPE) ASSERTION REASON|11 VideosSAMPLE PAPER 2023 TERM I
CBSE MODEL PAPER|Exercise SECTION E|7 Videos
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE QUESTION PAPER (CHEMISTRY )-SECTION B
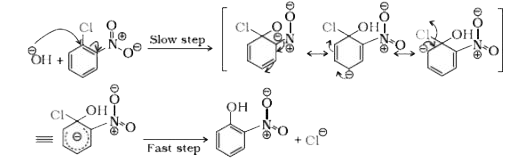
- With the help of resonating structures explain the effect of presence ...
Text Solution
|
- Carry out the following conversions in not more than 2 steps: (i)Ani...
Text Solution
|
- A glucose solution which boils at 101.04^(@)C at 1 atm. What will be r...
Text Solution
|
- (i) Write the electronic configuration iron ion in the following compl...
Text Solution
|
- (i) Predict the geometry of [NiCN(4)]^(2-) (ii) Calculate the spin on...
Text Solution
|
- For a reaction the rate law expression is represented as follows: Ra...
Text Solution
|
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- The C-14 content of an ancient piece of wood was found to have three t...
Text Solution
|
- When 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- Give the formula and describe the structure of a noble gas species whi...
Text Solution
|
- The following haloalkanes are hydrolysed in presence of aq KOH. (i)...
Text Solution
|
- Atoms of element P form ccp lattice and those of the element Q occupy ...
Text Solution
|
- Give reasons for the following: i. Transition elements act as cataly...
Text Solution
|