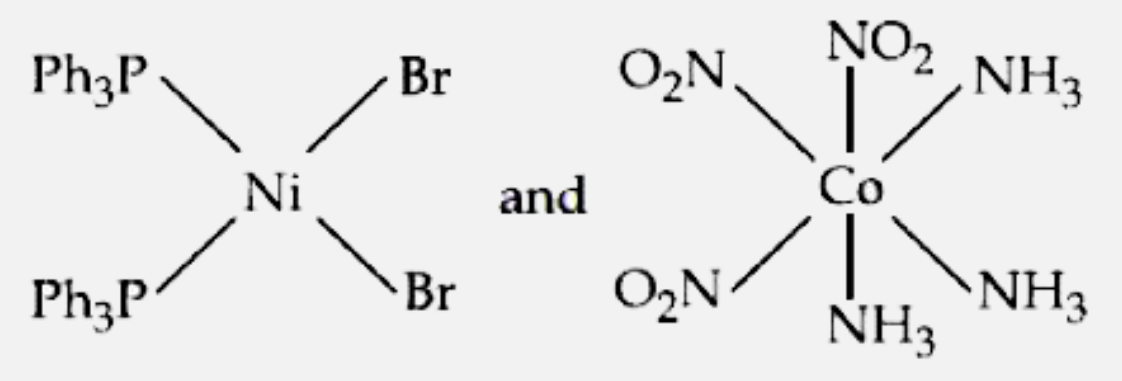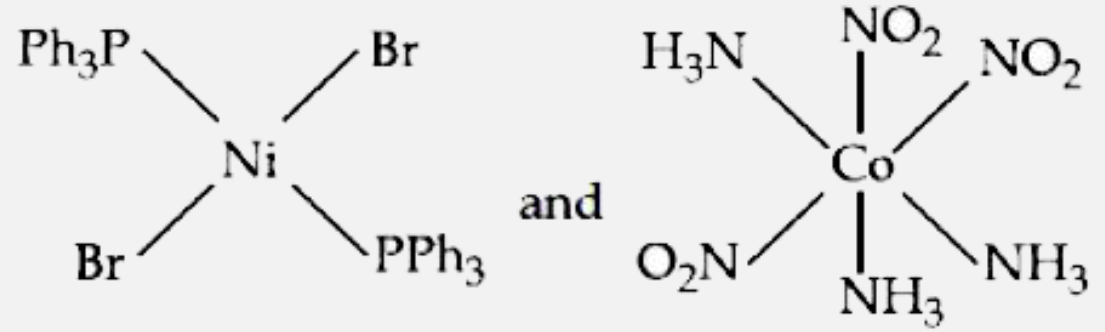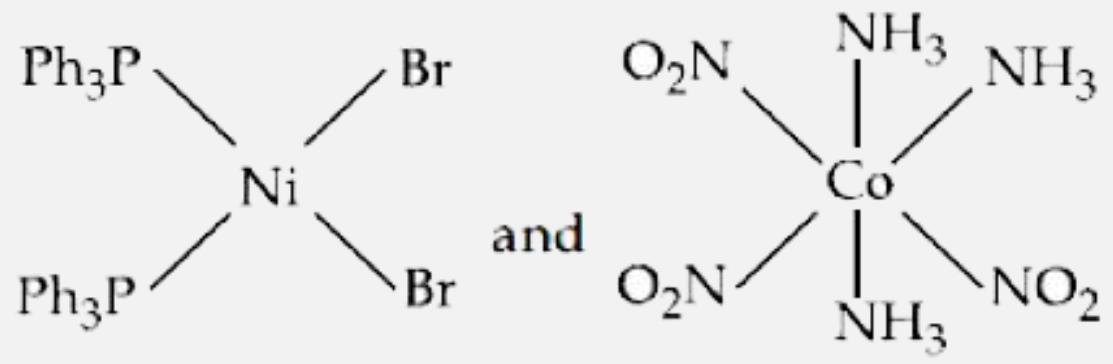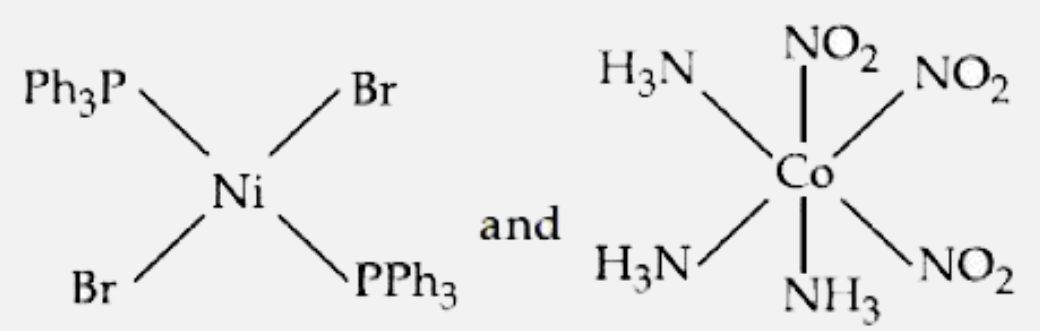A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY SECTION B|30 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise Chemistry (Section A)|20 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION B)|20 VideosJEE MAIN
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY|150 VideosJEE MAIN 2022
JEE MAINS PREVIOUS YEAR|Exercise Question|561 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2021-CHEMISTRY SECTION A
- The correct structures of trans- [NiBr2(PPh3)2] " and meridonial - " [...
Text Solution
|
- The chemical that is added to reduce the melting points of the reactio...
Text Solution
|
- Compound with molecular formula C3 H6 O can show :
Text Solution
|
- Match List - I with List - II Choose the most appropriate answer ...
Text Solution
|
- Match List - I with List - II Choose the most appopriate answer f...
Text Solution
|
- The ionic radius of Na^(+) ion is 1.02Å. The ionic (in A) of Mg^2 and ...
Text Solution
|
- In a binary compound, atoms of element A form a hcp structure and thos...
Text Solution
|
- Reaction of Grignard reagent, C2 H5 MgBr " with " C8 H8 O followed by ...
Text Solution
|
- Given below are two Statements : One is labelled as Assertion A and th...
Text Solution
|
- Considering the above reation, X and Y respectively are :
Text Solution
|
- Considering the above reation, identify the product ''X'' :
Text Solution
|
- Reagent, 1-naphthylamine and sulphanilic acid in acetic is used for th...
Text Solution
|
- Consider the above chemical reaction and identify product ''A'' :
Text Solution
|
- The statements that are TRUE : (A) methane leads to both global warm...
Text Solution
|
- The number of ionisable hydrogens present in the product obtained from...
Text Solution
|
- A cetain orbital has no angular nodes and two radial nodes. The orbita...
Text Solution
|
- Match List - I with List - II Choose the most appopriate answer f...
Text Solution
|
- A non-reducing sugar ''A'' hydrolyses to give two reducing mono sachha...
Text Solution
|
- Match List - I with List - II Choose the most appopriate match :
Text Solution
|
- Match List - I with List - II Choose the most appropriate match ...
Text Solution
|