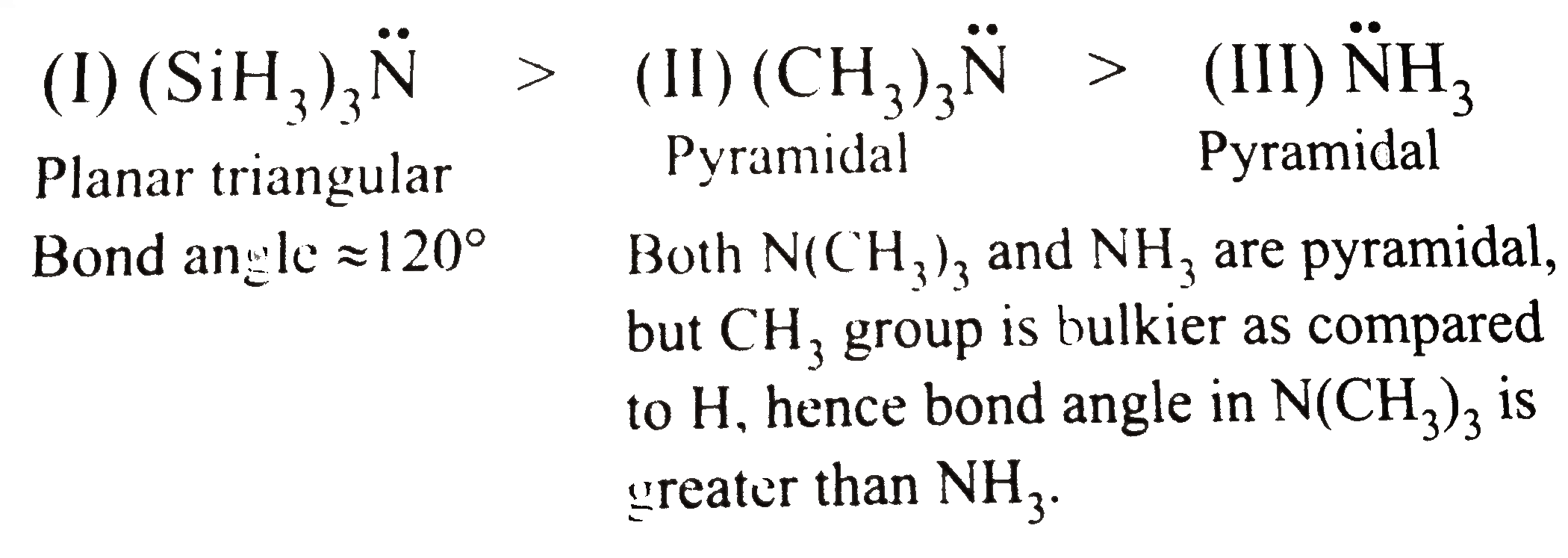A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|12 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct)|104 VideosP-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Application Exercises 2.1 (Objective)|10 VideosORGANIC COMPOUNDS WITH FUNCTIONAL GROUP
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Analytical And Descriptive|24 VideosP-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILY-Exercises (Linked Comprehension)
- NH3 has got pyramidal structure. By replacement of H atom it forms (CH...
Text Solution
|
- NH3 has got pyramidal structure. By replacement of H atom it forms (CH...
Text Solution
|
- NH3 has got pyramidal structure. By replacement of H atom it forms (CH...
Text Solution
|
- Solid N2 O5 exists as NO2^(oplus) NO3^(Ө) and hence is called nitroniu...
Text Solution
|
- Solid N2 O5 exists as NO2^(oplus) NO3^(Ө) and hence is called nitroniu...
Text Solution
|
- Solid N2 O5 exists as NO2^(oplus) NO3^(Ө) and hence is called nitroniu...
Text Solution
|
- PCl5 has trigonal pyramidal geometry with sp^3 d hybridisation in gase...
Text Solution
|
- PCl5 has trigonal pyramidal geometry with sp^3 d hybridisation in gase...
Text Solution
|
- PCl5 has trigonal pyramidal geometry with sp^3 d hybridisation in gase...
Text Solution
|
- In solid state PCl(5) exist as [PCl(4)]^(+) [PCl(6)]^(-). The hybridis...
Text Solution
|
- The pronounced change from non-metallic behaviour and also increase in...
Text Solution
|
- The pronounced change from non-metallic behaviour and also increase in...
Text Solution
|
- Which of the following fluorides does not exist?
Text Solution
|
- Which of the following oxides is most acidic ?
Text Solution
|
- The pronounced change from non-metallic behaviour and also increase in...
Text Solution
|
- The pronounced change from non-metallic behaviour and also increase in...
Text Solution
|
- The pronounced change from non-metallic behaviour and also increase in...
Text Solution
|
- Phosphorus forms a number of oxoacids which differ in their structures...
Text Solution
|
- Phosphorus forms a number of oxoacids which differ in their structures...
Text Solution
|
- Phosphorus forms a number of oxoacids which differ in their structures...
Text Solution
|