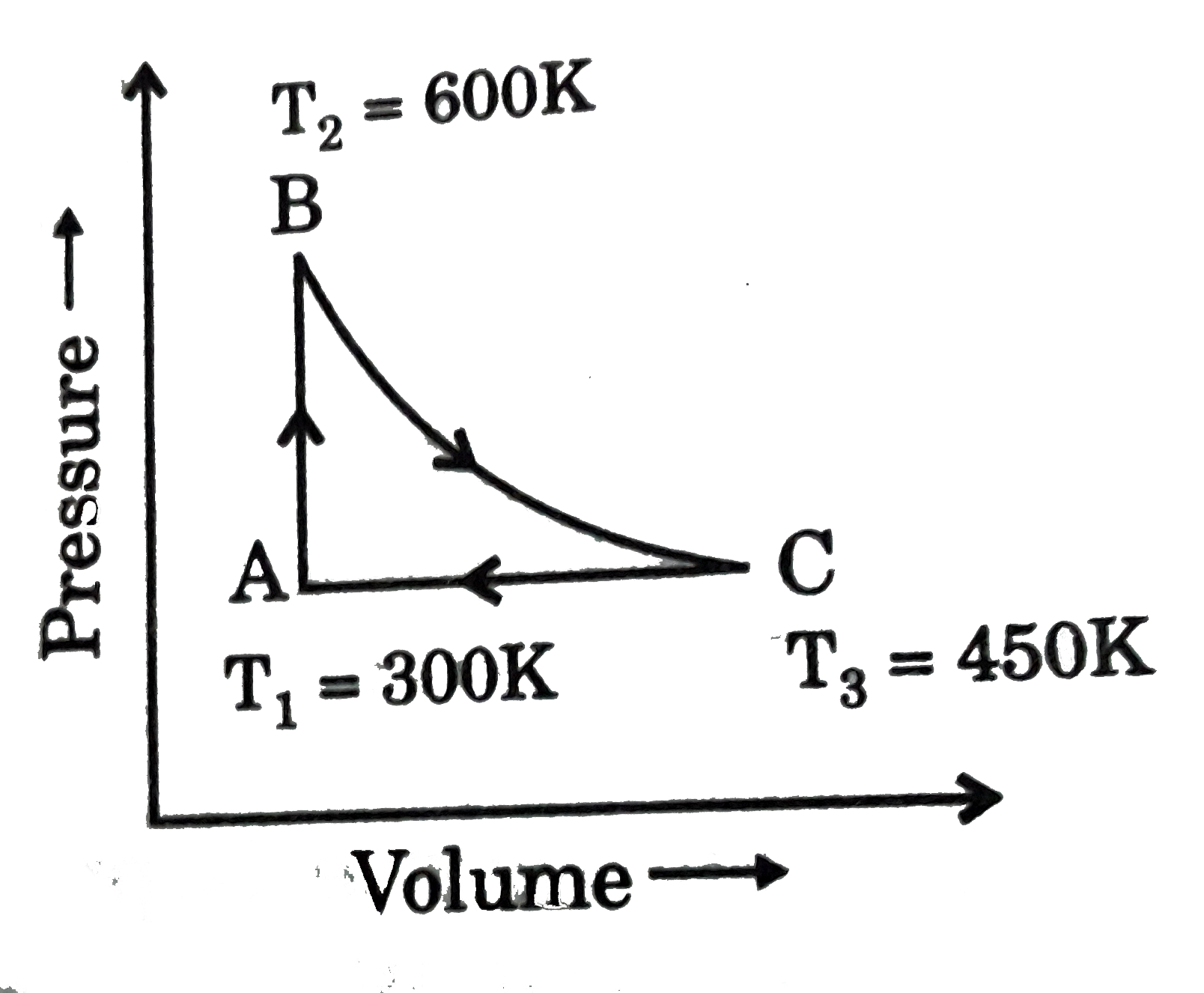
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise Exercise|50 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (Section - A) Objective Type Questions|55 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-I|9 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-SECTION-J
- A heat engine carries one mole of an ideal monoatomic gas around the c...
Text Solution
|
- Calculate the average molar heat capacity at constant volume of a mix...
Text Solution
|
- What is the change in entropy when 2.5 mole of water is heated from 27...
Text Solution
|
- Which amog the following is most soluble in water?
Text Solution
|
- A hungry man weighing 80 kg take quickly 20 g lunck, and then climbs ...
Text Solution
|
- According to second law of thermodynamics
Text Solution
|
- Consider the reacton C(s) + (1)/(2) O(2)(g) rarr CO(g) + 200 kJ Th...
Text Solution
|