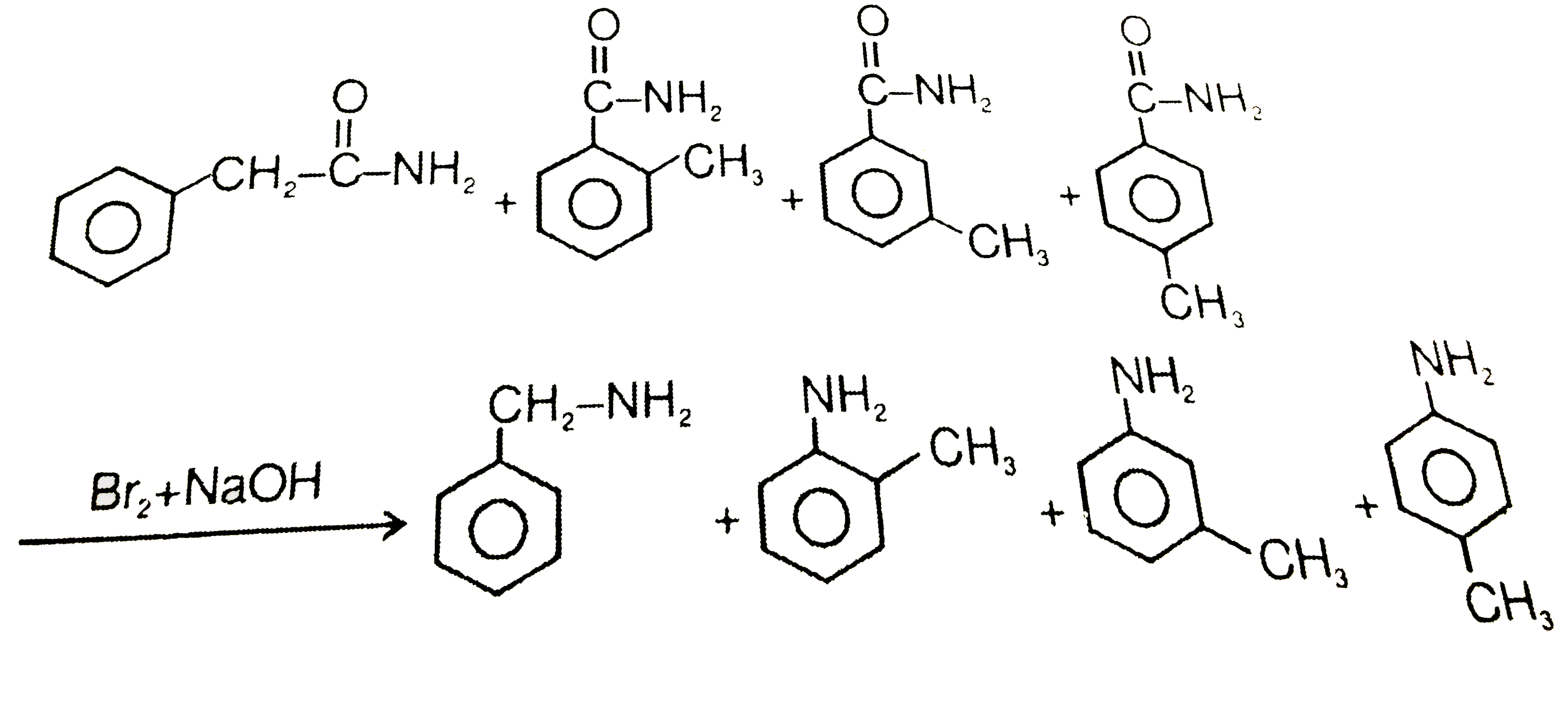
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - III Practice Test-19|1 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - III Practice Test-20|1 VideosAROMATIC COMPOUNDS
RESONANCE ENGLISH|Exercise Part - III Practice Test-17|1 VideosALKYL HALIDE, ALCOHOL, PHENOL, ETHER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|56 VideosBASIC CONCEPTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems