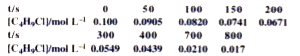Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ICSE|Exercise INTEXT QUESRTIONS|81 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS)A.FILL IN THE BLANKS) |54 VideosCHEMICAL KINETICS
ICSE|Exercise ISC EXAMINATION QUESTIONS (NUMERICAL PROBLEMS )|57 VideosBIOMOLECULES
ICSE|Exercise ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)|21 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL KINETICS-PROBLEM
- From the data given below, calculate the average rate of the reaction ...
Text Solution
|
- When 50 mL of 2M solution of N2 O5 was heated, 0.28 L of O2 at NTP was...
Text Solution
|
- Consider the following reaction which proceeds in a closed vessel. ...
Text Solution
|
- Express the rate of following reactions PCl5 to PCl3 +Cl2
Text Solution
|
- Express the rate of following reactions 2NO2 to 2NO +O2
Text Solution
|
- Express the rate of following reactions. H2 +I2 hArr 2HI
Text Solution
|
- Express the rate of following reactions. N2 +3H2 hArr 2NH3
Text Solution
|
- Identify the reaction order from each of the following rate constants ...
Text Solution
|
- Nitrogen dioxide (NO2) reacts with fluorine (F2) to yield nitryl fluor...
Text Solution
|
- Nitrogen dioxide (NO2) reacts with fluorine (F2) to yield nitryl fluor...
Text Solution
|
- Nitrogen dioxide (NO2) reacts with fluorine (F2) to yield nitryl fluor...
Text Solution
|
- For the reaction, X + Y to Z, the rate is given as k[X]^(1//3) [Y]^(...
Text Solution
|
- Carbonyl chloride gas decomposes to give carbon monoxide gas and chlor...
Text Solution
|
- The reaction N2O5 to 2NO2 + 1/2 O2 is of first order in N2 O5. Its ra...
Text Solution
|
- Consider the following first order reaction. 2H2 O2 (aq) overset(I^...
Text Solution
|
- Consider the following first order reaction. 2H2 O2 (aq) overset(I^...
Text Solution
|
- For the reaction 2X+Y+Zto X2 YZ, the rate equation is : Rate =k [X] [Y...
Text Solution
|
- For the reaction 2X+Y+Zto X2 YZ, the rate equation is : Rate =k [X] [Y...
Text Solution
|
- A substance decomposes following first order kinetics. If the half-lif...
Text Solution
|
- The rate constant of a first order reaction is 2.31 xx 10^(-2) s^(-1)....
Text Solution
|