Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
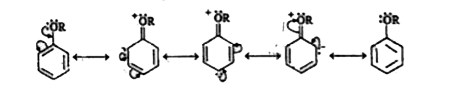
BETTER CHOICE PUBLICATION-ALCOHOLS , PHENOLS AND ETHERS -11 . 6 . 3 . CHEMICAL PROPERTIES
- Explain the fact that in aryl ethers (i) the alkoxy group activates ...
Text Solution
|
- Write the mechanism of the reaction of HI with methoxymethane .
Text Solution
|
- How diethyl ether reacts with HI
Text Solution
|
- How diethyl ether reacts with PCl(5)
Text Solution
|
- How diethyl ether reacts with Cl(2)
Text Solution
|
- Why are ethers relatively inert compounds ?
Text Solution
|
- Phenyl methyl ether reacts with HI to give phenol and methylIodide and...
Text Solution
|
- How do you account for the miscibility of ethoxy ethane with water.
Text Solution
|