Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 104-CHEMISTRY (SUBJECTIVE NUMERICAL)
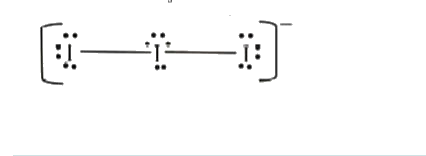
- The total number of lone pairs of electrons in I(3)^(-) ion is ..........
Text Solution
|
- Lithium hydride is used in the synthesis of other hydrides. LiH +B(...
Text Solution
|
- Methyl cyanide underset("conc. "HCl)overset(2[H],SnCl(2))to P overset...
Text Solution
|
- 'X' moles of conc. hydroiodic acid react with acetamide in the presenc...
Text Solution
|
- How many alcohols from the following set gives geometric isomers on de...
Text Solution
|
- What is the pH of the resulting solution when equal volumes of 0.1 M N...
Text Solution
|
- In the Freundlich adsorption isotherm, the value of ((1)/(n)) is betw...
Text Solution
|
- A metal crystallises in a simple cubic unit cell. If the length of the...
Text Solution
|
- The maximum number of electrons in a subshell with n = 4 and l=3 is ....
Text Solution
|
- Consider a first order gas phase decomposition reaction given below: A...
Text Solution
|