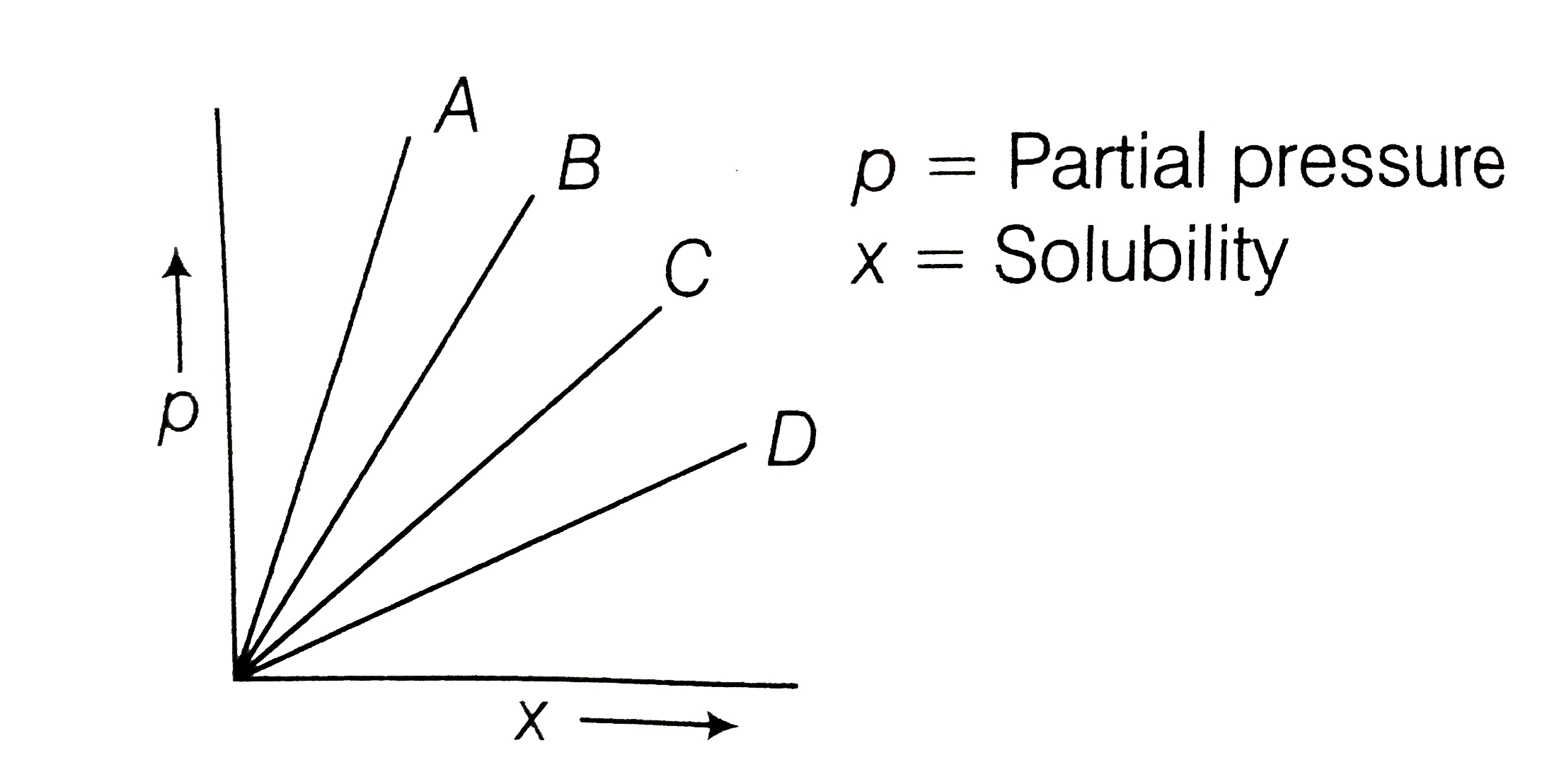A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-PRACTICE SET 19-Paper 1 (Physics & Chemistry)
- At constant temperature, on the basis of given graph which gas possess...
Text Solution
|
- According to third law of thermodynamics, the entropy at 0 K is zero f...
Text Solution
|
- Oxidation state of nitrogen is corectly given for {:(,,"Compound",,,...
Text Solution
|
- Milk turns sour at 40^(@)C three times as faster as at 0^(@)C. The ene...
Text Solution
|
- How many mL of perhydrol is requried to produce sufficient oxygen whic...
Text Solution
|
- Nitric acid oxidise phosphrous to
Text Solution
|
- The order of electron affinity among halogen is
Text Solution
|
- Which of the following oxides of chromium is amphoteric in nature?
Text Solution
|
- Which of the following oxides of chromium is amphoteric in nature?
Text Solution
|
- The correct order of boiling point is
Text Solution
|
- Which physical property in the alkali metal group increases with atomi...
Text Solution
|
- The colour of sky is due to
Text Solution
|
- The standard emf for the cell cell reaction Zn + Cu^(2+) rarr Zn^(2+)...
Text Solution
|
- What is the equivalent weight of "SnCl"(2) in the following reaction ?...
Text Solution
|
- What type of crystal defect is indicated in the diagram given below : ...
Text Solution
|
- Copper is refined by
Text Solution
|
- Dinitrogen pentoxide (N(2)O(5)), a colourless solid is prepared by
Text Solution
|
- The shape of "XeOF"(2) on the basis of VSEPR theory is
Text Solution
|
- All alkyl halides undergo reduction process except
Text Solution
|
- Mark the correct statement about aldehydes and ketones
Text Solution
|