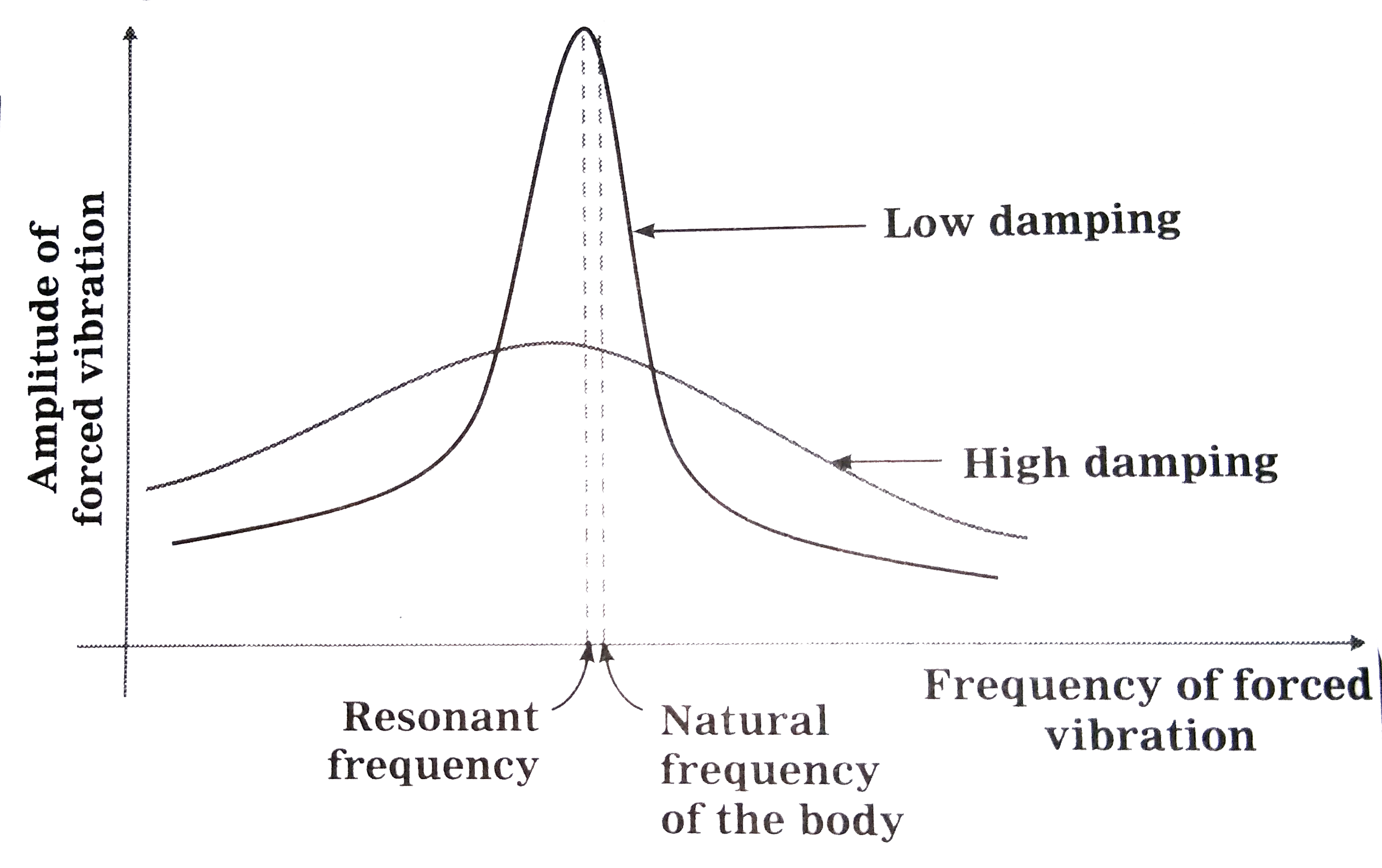
Text Solution
Verified by Experts
Topper's Solved these Questions
EXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Kinetic Theory of Gases and Radiation|4 VideosEXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Wave Theory of light|3 VideosEXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Wave Motion|2 VideosEXPERIMENTS AND DIAGRAMS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise ATOMS, MOLECULES AND NUCLEI|1 VideosINSTRUMENTS : CONSTRUCTION AND WORKING
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Semiconductors|6 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-EXPLANATION, CHARACTERISTICS AND PROPERTIES -Stationary Waves
- State the principle of superposition of waves.
Text Solution
|
- With neat labelled diagrams, explain the different modes of vibration ...
Text Solution
|
- Show that all harmonics are present on a stretched string between two ...
Text Solution
|
- What are forced vibrations and resonance ? Show that only odd harmonic...
Text Solution
|
- Resonance is an example of
Text Solution
|