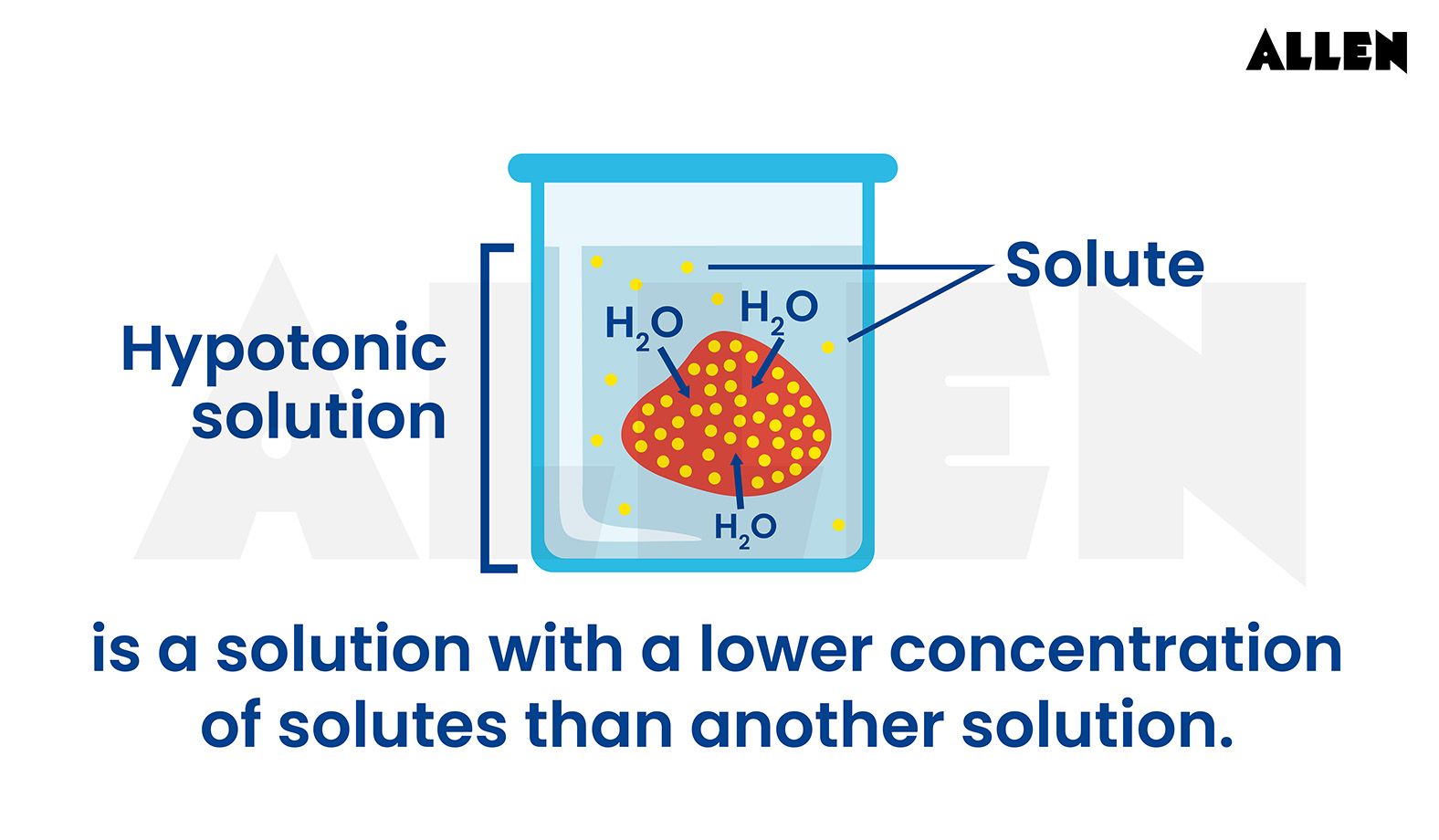
Hypotonic Solution
1.0Tonicity
Refers to the relative concentration of solutes in two solutions separated by a semipermeable membrane. Tonicity is determined by the relative concentration of membrane-impermeable solutes on either side of a selectively permeable cell membrane, influencing the direction and magnitude of osmotic flux. There are three main types of tonicity: hypertonic, hypotonic, and isotonic.
2.0Hypotonic Solution Definition
A hypotonic solution meaning involves a lower concentration of solutes compared to another solution, a term frequently applied in biology to describe the environment outside a cell.
Hypotonic Solution
Specifically, a solution is deemed hypotonic when its solute concentration is lower than that of the cell's cytosol. This disparity in solute concentrations sets the stage for osmotic pressure to come into play, leading to the movement of water into the cell. Consequently, the cell often exhibits a turgid or bloated appearance.
3.0Hypotonic Solution Example
Hypotonic solution on different cell types:
In the context of cells lacking a cell wall, such as animal cells, exposure to a hypotonic solution with a substantially lower solute concentration than the cell's cytosol results in the uptake of excess water.
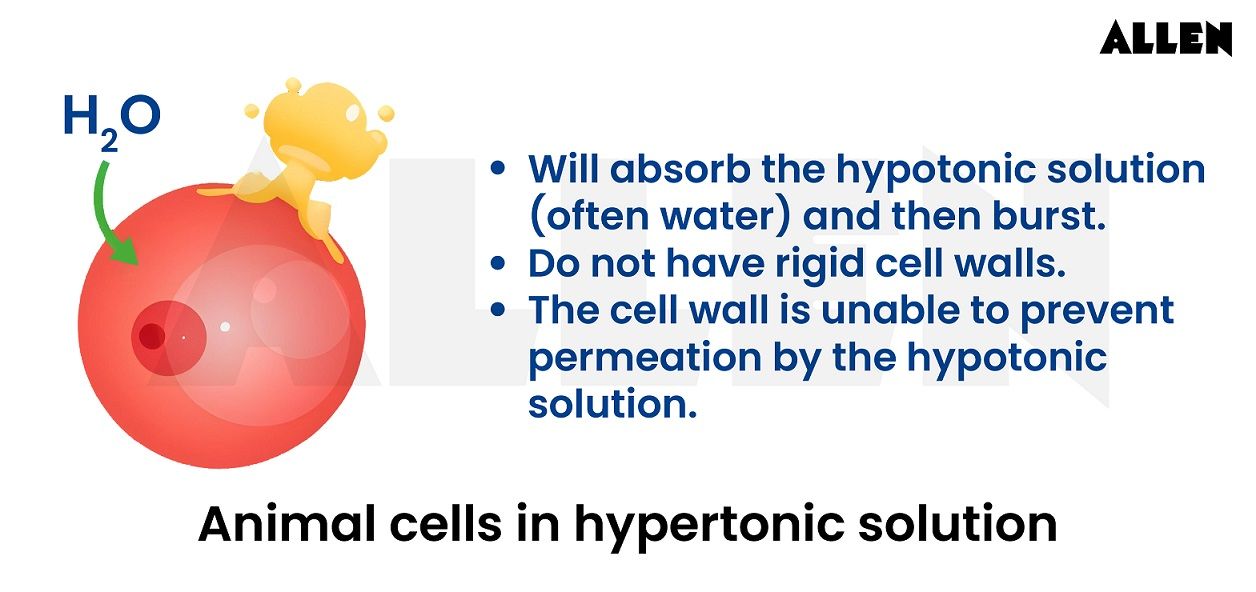
This influx of water into the cell leads to cytolysis, a process where the cell membrane ruptures due to the swelling caused by the increased water volume.
When plant cells are exposed to a hypotonic solution, a distinct set of responses occurs. The central vacuole within the plant cell absorbs the extra water, creating turgor pressure.
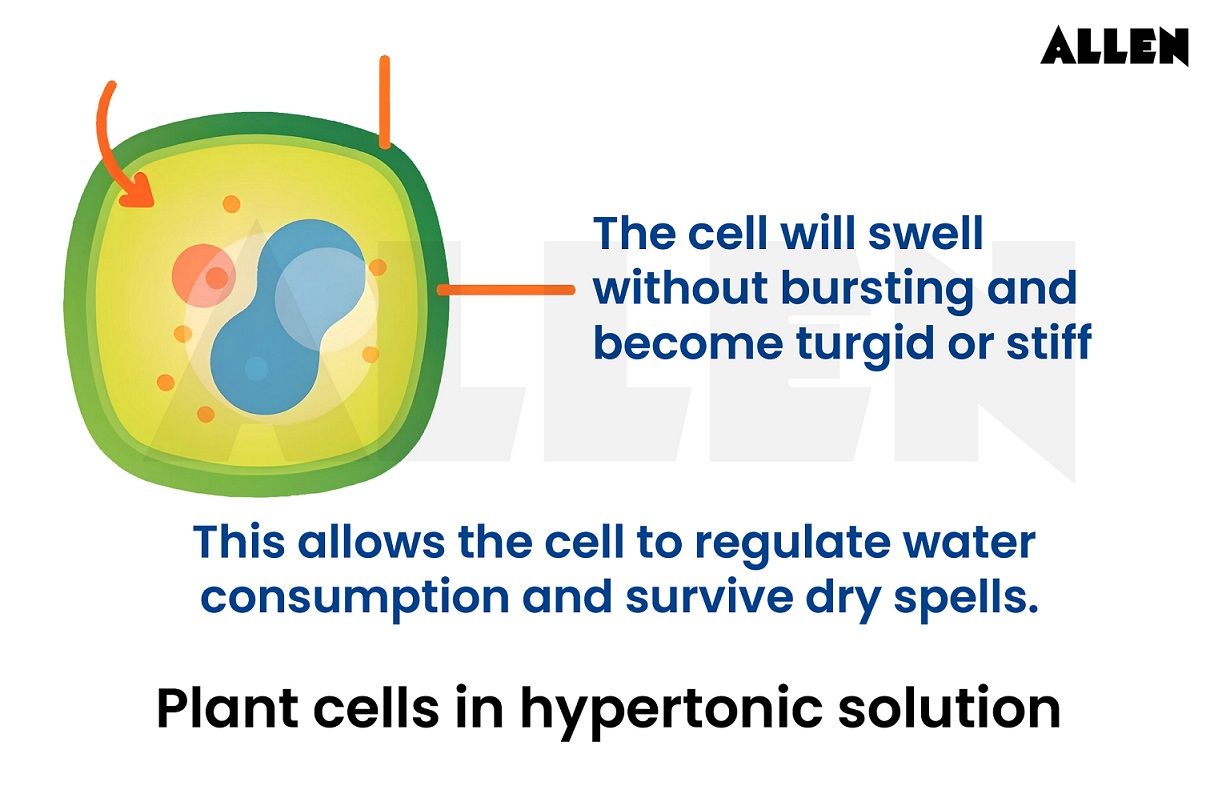
This pressure exerts force on the cell membrane, pushing it against the rigid cell wall. The interplay between the internal components of the cell and the external hypotonic environment is evident in the maintenance of cell shape and stability. The rigid cell wall acts as a counterforce, preventing the plant cell from bursting even as it takes on additional water.
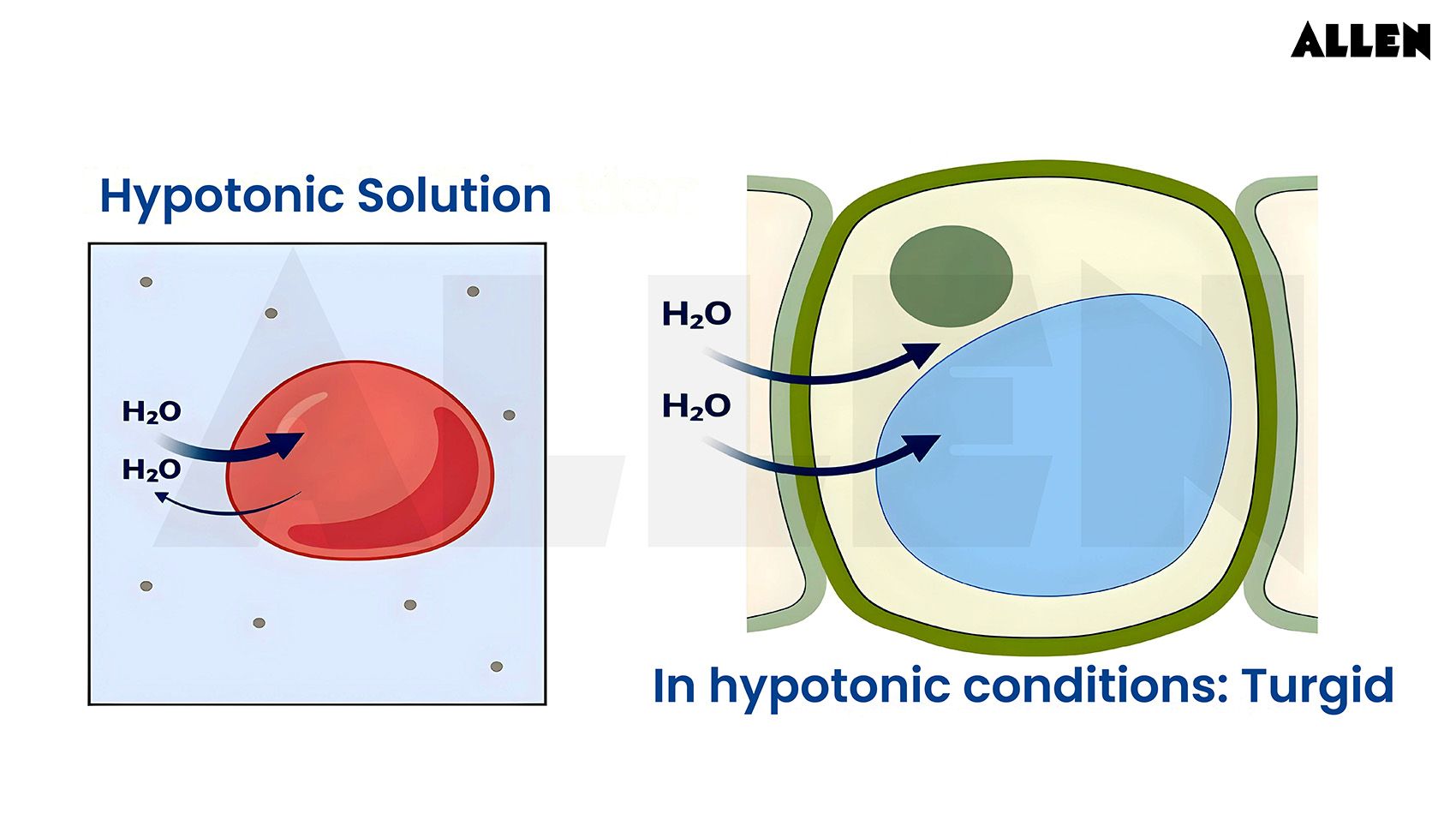
4.0Hypotonic Solution Figure
Frequently Asked Questions
Join ALLEN!
(Session 2025 - 26)