A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNIL BATRA (41 YEARS IITJEE PHYSICS)-HEAT AND THERMODYNAMICS-JEE Main And Advanced
- The graph, shown in the adjacent diagram, represents the variation of ...
Text Solution
|
- Two rods, one of aluminium and the other made of steel, having initial...
Text Solution
|
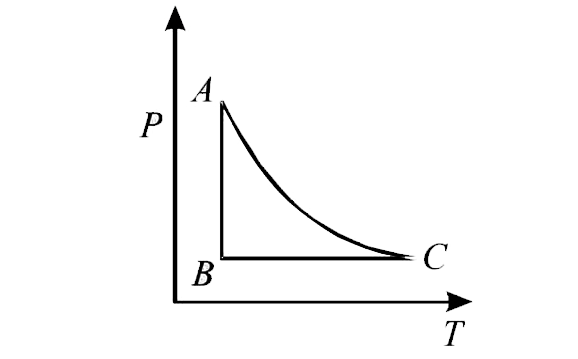
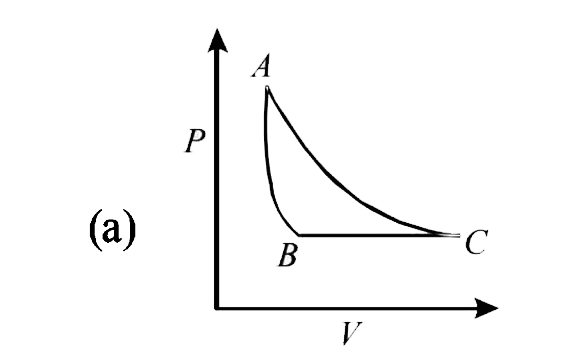
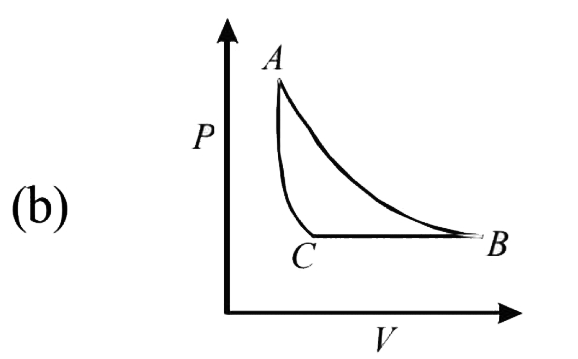
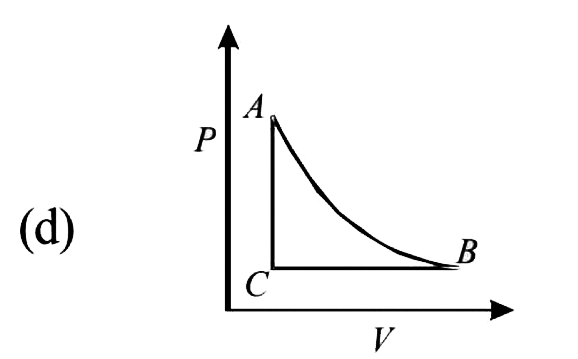
- The PT diagram for an ideal gas is shown in the figure, where AC is an...
Text Solution
|
- 2kg of ice at 20^C@ is mixed with 5kg of water at 20^C@ in an insulati...
Text Solution
|
- Three discs A,B and C having radii 2,4 and 6 cm respectively are coate...
Text Solution
|
- If liquefied oxygen at 1 atmospheric pressure is heated from 50K to 30...
Text Solution
|
- Two identical rods are connected between two containers on of tehm is ...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands to P2,V2 and then compre...
Text Solution
|
- Variation of radiant energy emitted by sun, filament of tungsten lamp ...
Text Solution
|
- In which of the following process, convection does not take place pri...
Text Solution
|
- A spherical body of area A, and emissivity e = 0.6 is kept inside a bl...
Text Solution
|
- Calorie is defined as the amount of heat required to raise temperature...
Text Solution
|
- Water of volume 2 litre in a container is heated with a coil of 1kW at...
Text Solution
|
- Water is filled up to a height h in a beaker of radiys R as shown in t...
Text Solution
|
- An ideal gas is expanding such that PT^@=constant. The coefficient of ...
Text Solution
|
- A real gas behaves like an ideal gas if its
Text Solution
|
- 5.6 liter of helium gas at STP is adiabatically compressed to 0.7 lite...
Text Solution
|
- A mixture of 2 moles of helium gas (atomic mass=4amu) and 1 mole of ar...
Text Solution
|
- Two moles of ideal helium gas are in a rubber balloon at 30^@C. The ba...
Text Solution
|
- Two rectangular blocks, having identical dimensions, an be arranged ei...
Text Solution
|