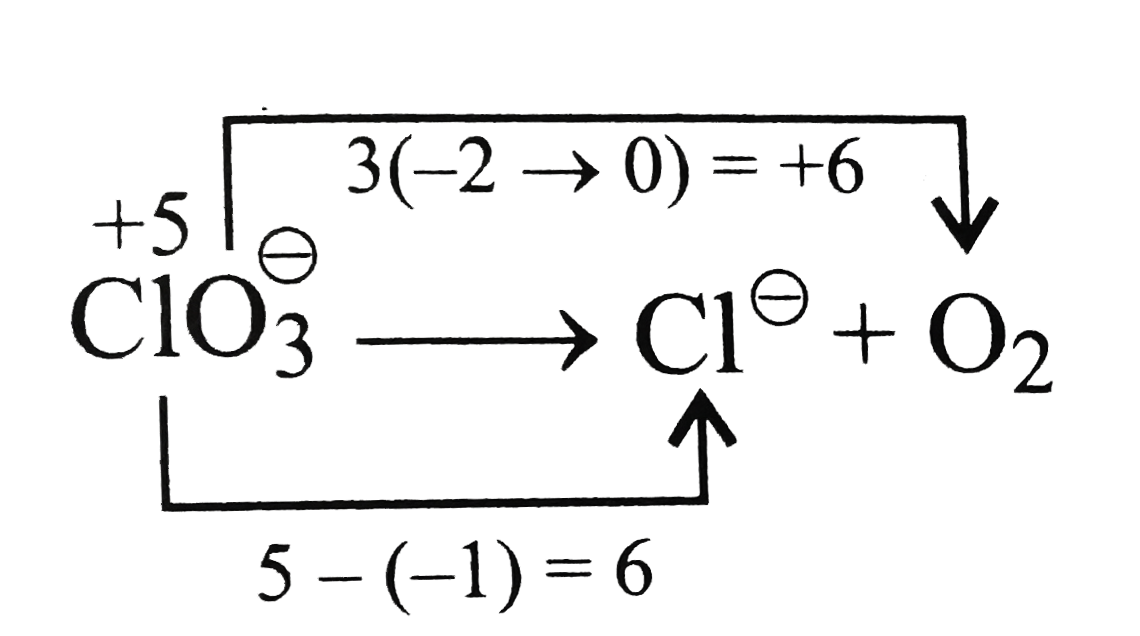
Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Ex 3.3|13 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Ex 3.4 (A)|7 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Ex 3.1|3 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|23 Videos
Similar Questions
Explore conceptually related problems