A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
A2Z|Exercise Stoichiometry In Redox Reactions|44 VideosREDOX REACTIONS
A2Z|Exercise Type Of Redox Reaction And Equivalent Weight|42 VideosREDOX REACTIONS
A2Z|Exercise Section D - Chapter End Test|29 VideosP BLOCK ELEMENTS ( Group 13 -14)
A2Z|Exercise Section D - Chapter End Test|30 VideosS BLOCK ELEMENTS ( GROUP 13 - 14)
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-REDOX REACTIONS-Balancing Of The Equation
- For the redox reaction xFe^(2+)+yCr(2)O(7)^(2-)+zH^(+) rarr Fe^(3+)+...
Text Solution
|
- C(2)H(6)(g)+nO(2) rarr CO(2)(g)+H(2)O(l) In this equation, the ratio...
Text Solution
|
- Number of electron involved in the reduction of Cr(2)O(7)^(2-) ion in ...
Text Solution
|
- 2MnO(4)^(-)+5H(2)O(2)+6H^(-) rarr 2Z+5O(2)+8H(2)O. In this reaction Z ...
Text Solution
|
- H(2)O can be oxidised to
Text Solution
|
- When ZnS is boiled with strong nitric acid, the products are zinc nitr...
Text Solution
|
- Which of the following equations is a balanced one?
Text Solution
|
- In the following reaction 2I- + Cr(2)O(7)^(2-)+14H^(+) rarr I(2)+2Cl...
Text Solution
|
- For the redox reaction Cr(2)O(7)^(-2)+H^(+)+Ni rarr Cr^(3)+Ni^(2+)+H...
Text Solution
|
- MnO(4)^(-) oxidises H(2)O(2) to O(2) in acidic medium xMnO(4)^(-)+yH...
Text Solution
|
- What is the molecular state of sulphur as reactant in, sulphur +12OH^(...
Text Solution
|
- In the following balanced reaction, 4O(2)^(x)+2H(2)O rarr 4OH^(-)+3O...
Text Solution
|
- In balancing the half reaction CN^(ө)rarrCNO^(ө)(skeltan) The numb...
Text Solution
|
- In the following equation: CIO(3)^(-)+6H^(+)+.XrarrCl^(-)+3H(2)O, th...
Text Solution
|
- I^(-) reduces IO(3)^(-) and I(2) and itself oxidised to I(2) in acidic...
Text Solution
|
- In the reaction xHI+yHNO(3) rarr NO+I(2)+H(2)O
Text Solution
|
- Balance the following equation stepwise: Cr(2)O(7)^(2-) + Fe^(2+)++H...
Text Solution
|
- Values of p, q, r, s and t are in the following redox reaction pBr(2...
Text Solution
|
- In the following reaction: x KMnO(4)+y NH(3) rarr KNO(3)+MnO(2)+KOH+...
Text Solution
|
- CuS is dissolved in dil. HNO(3). Balanced equation with correct produc...
Text Solution
|
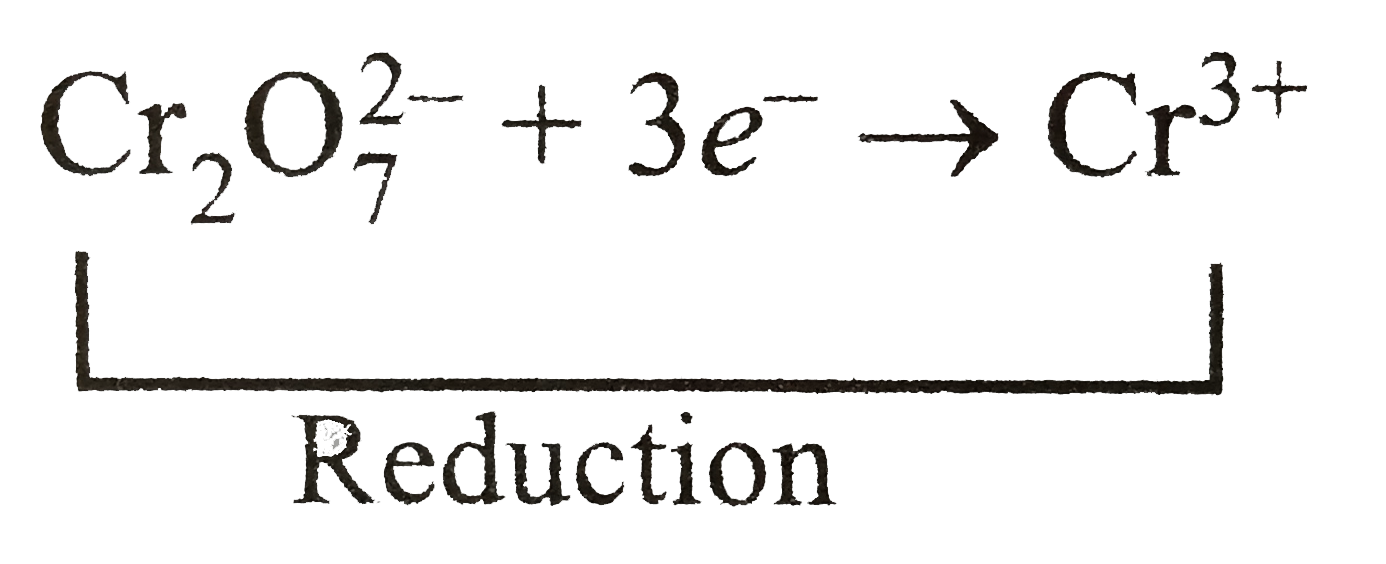 In this reaction three electrons are required for the reduction of `Cr_(2)O_(7)^(2-)` into `Cr^(3+)`.
In this reaction three electrons are required for the reduction of `Cr_(2)O_(7)^(2-)` into `Cr^(3+)`.