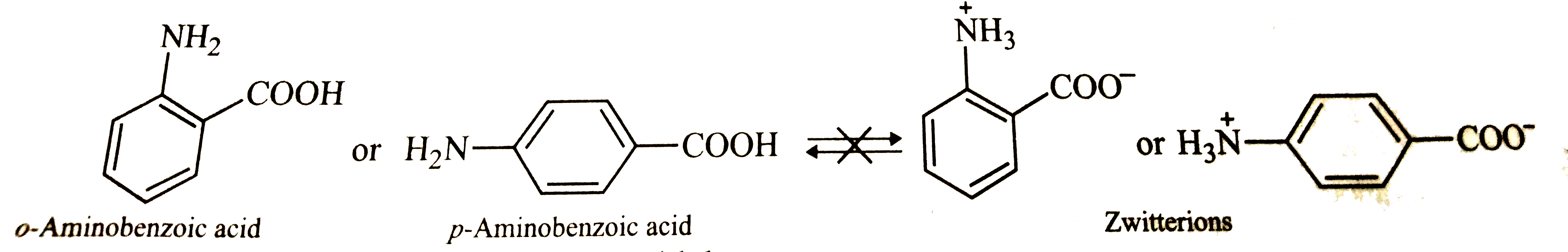Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
PRADEEP|Exercise NCERT (QUESTIONS AND EXERCISES) WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)|8 VideosBIOMOLECULES
PRADEEP|Exercise NCERT (EXERCISES)|25 VideosBIOMOLECULES
PRADEEP|Exercise TEST YOUR GRIP (FILL IN THE BLANKS )|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 VideosCHEMICAL KINETICS
PRADEEP|Exercise ADVANCED PROBLEMS FOR COMPETITIONS|14 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-BIOMOLECULES -Conceptual Questions
- What are reducing and non-reducing sugars ? What is the structural fea...
Text Solution
|
- Fructose contains a keto group but still it reduces Tollen's reagent. ...
Text Solution
|
- Glucose does not give 2,4- DNP test , Schiff's test or does not give...
Text Solution
|
- Name two components of starch. How do they differ from each other stru...
Text Solution
|
- Glycine exists as NH(3)""^(+)CH(2)COO^(-) , zwitter ion but anthranili...
Text Solution
|
- Can the acid chloride of an alpha-amino acid be made by treating it wi...
Text Solution
|
- Is a diet consisting mainly of rice an adequate diet? Why or why not?
Text Solution
|
- What is the monomer unit of protein ? Give two examples of monomers , ...
Text Solution
|
- Give reason for the following : (i) Amino acids have high melting poin...
Text Solution
|
- Towards which electrode would an alpha-amino acid migrate in an electr...
Text Solution
|
- What changes occur in the nature of egg proteins on boiling ?
Text Solution
|
- Define enzymes ? What is the most important reason for their specific ...
Text Solution
|
- What is the difference between hormones and vitamins ?
Text Solution
|
- If one of the strands of DNA has the following sequence of bases in th...
Text Solution
|