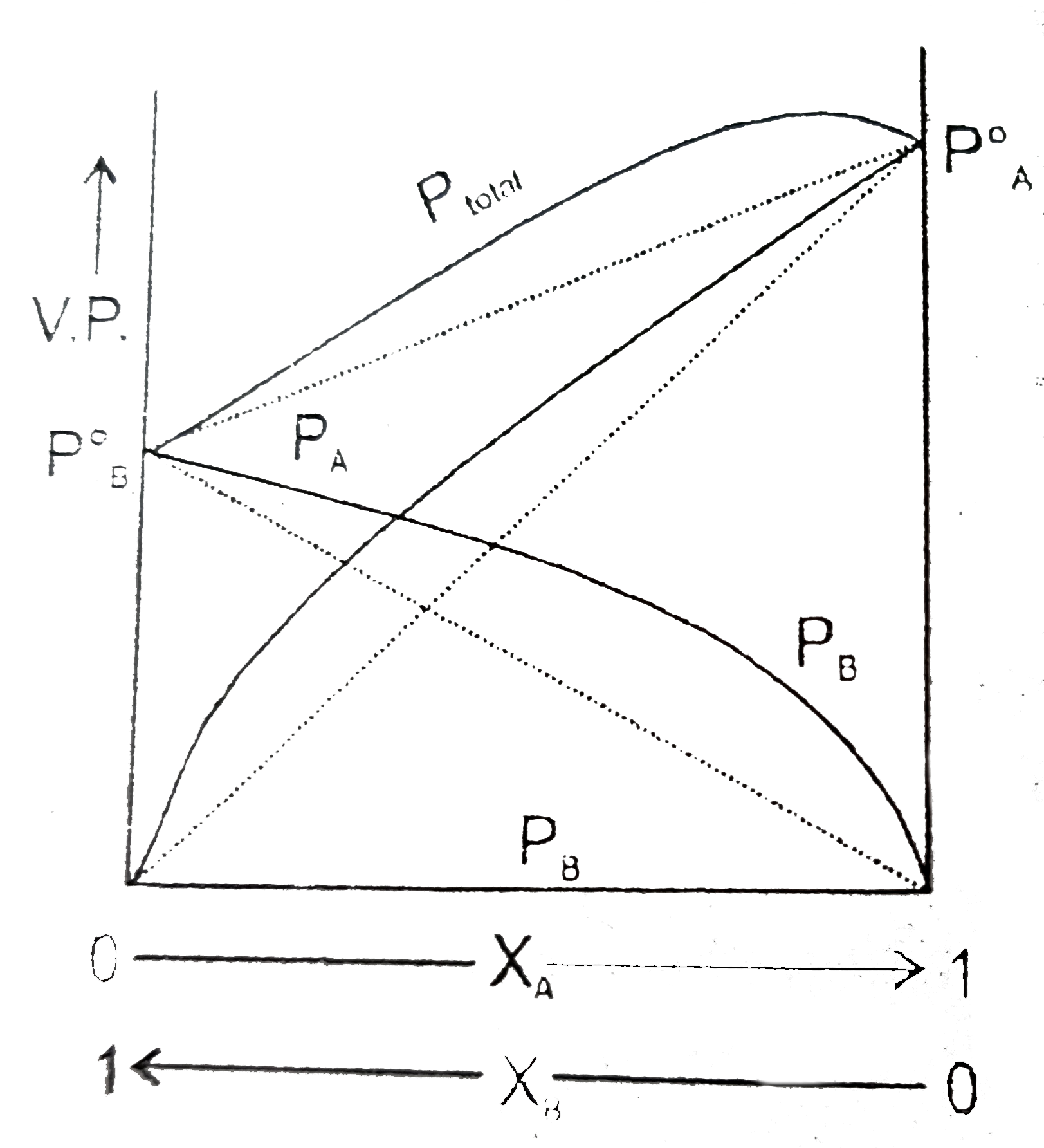
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
RESONANCE|Exercise EXERCISE-3(PART-1)|14 VideosSOLUTIONS
RESONANCE|Exercise EXERCISE-3(PART-2)|16 VideosSOLUTIONS
RESONANCE|Exercise EXERCISE-2(PART-2)|37 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLUTIONS-EXERCISE-2(PART-4)
- Answer the question (given below) which are based on the following dia...
Text Solution
|
- Answer the question (given below) which are based on the following dia...
Text Solution
|
- Addition of non-volatile solute to a solvent always inceases the colli...
Text Solution
|
- Addition of non-volatile solute to a solvent always inceases the colli...
Text Solution
|