A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Match the column type|6 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Multiple objective type|38 VideosCOORDINATION COMPOUNDS
GRB PUBLICATION|Exercise SBJECTIVE TYPE|98 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Subjective type|39 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-D-BLOCK ELEMENTS-Comprehension Type
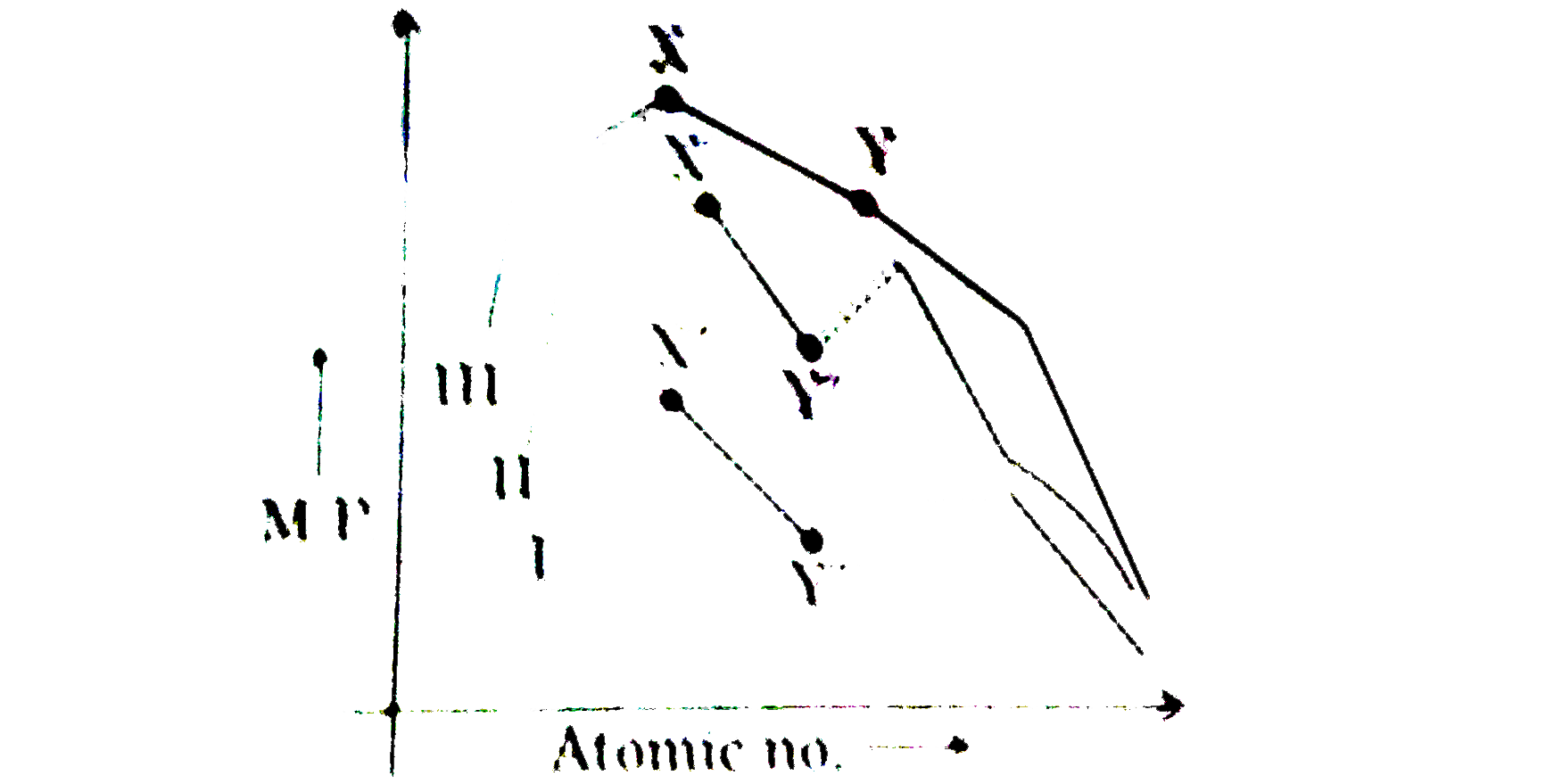
- Refer to the graph for trends in M.P. of transition elements of 3d,4d ...
Text Solution
|
- Refer to the graph for trends in M.P. of transition elements of 3d,4d ...
Text Solution
|
- Refer to the graph for trends in M.P. of transition elements of 3d,4d ...
Text Solution
|
- Transition metal and their compounds are used as catalysts in inductry...
Text Solution
|
- Transition metal and their compounds are used as catalysts in inductry...
Text Solution
|
- Transition metal and their compounds are used as catalysts in inductry...
Text Solution
|
- Transition metal and their compounds are used as catalysts in inductry...
Text Solution
|
- (X) is very important laboratory reagent which is prepared by its natu...
Text Solution
|
- (X) is very important laboratory reagent which is prepared by its natu...
Text Solution
|
- Due to availability of vacant orbitals of sufficiently low energy, d-b...
Text Solution
|
- Due to availability of vacant orbitals of sufficiently low energy, d-b...
Text Solution
|
- Due to availability of vacant orbitals of sufficiently low energy, d-b...
Text Solution
|