Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise SECTION-G INTEGER ANSWER TYPE QUESTIONS|8 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise SECTION-H MULTIPLE TRUE-FALSE TYPE QUESTION|2 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise SECTION-D LINKED COMPREHENSION TYPE QUESTIONS|6 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE|Exercise Assignment(Section-D)(Assertion - Reason Type Questions)|12 VideosPOLYMERS
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION-D)|13 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES-SECTION-E ASSERTION-REASON TYPE QUESTIONS
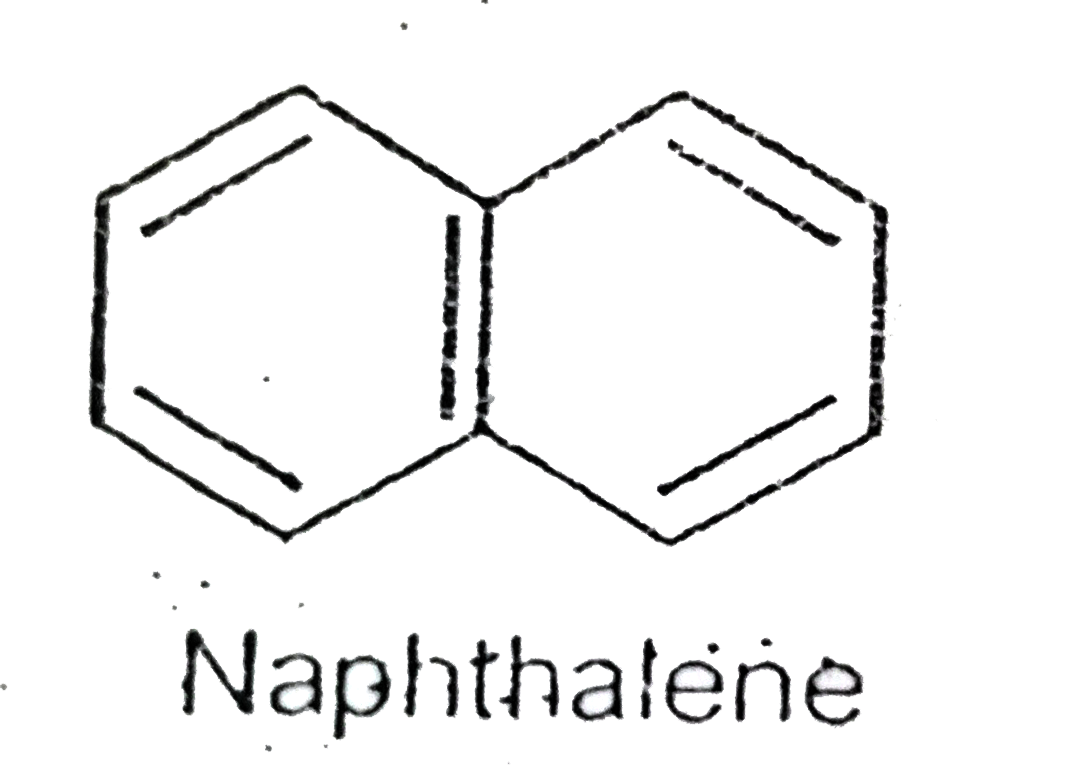
- Statement-1: In naphthalene all C-C bonds are equal and Stateme...
Text Solution
|
- Statement-1: p-Nitroaniline is more polar than nitrobenzene and St...
Text Solution
|
- Statement-1: All C-C bonds are equal in [10]-Annulene. and Stateme...
Text Solution
|
- Statement-1: and Statement-2: Conjugate base of phenol is resonan...
Text Solution
|
- Statement-1: A compound with odd number of nitroen always contains odd...
Text Solution
|
- Statement-1: Aldehydes and ketones having same molecular formulae are ...
Text Solution
|
- Statement-1: Carbocationic rearrangement is known as electrophilic rea...
Text Solution
|
- Statement-1: Cyclopentanone exhibits keto enol tautomerism. and St...
Text Solution
|
- Statement-1: CH(3)CH(2)overset(o+)(CH(2)) is less stable than CH(3)-un...
Text Solution
|
- Statement-1: Cyclopentanone exhibits keto enol tautomerism. and St...
Text Solution
|
- Statement-1: Keto form is less stable than enol form and Statemen...
Text Solution
|
- Statement-1: Aniline undergoes-Friedel craft alkylation more readily t...
Text Solution
|
- Statement-1: Bridge head carbocation is less stable than Bridge head c...
Text Solution
|