HEAT
"Taking a temperature. The thermometer provides a quantitative measure of how hot or cold things are and a basis for comparing your current temperature to your normal body temperature."
1.0Introduction
In this chapter, you will learn about the kind of energy that warms things i.e. heat. We get heat from different sources including Sun. You will learn that fuel is something that can be burned for heat and energy, such as wood, coal, charcoal and gasoline.
The measurement of temperature is a part of everyday life. We measure the temperature of the air outdoors to decide how to dress when going outside. For example, we wear woolen clothes during winters when it is cold outside. Woolen clothes keep us warm. We prefer to wear light coloured cotton clothes when it is hot. These give us a feeling of coolness. Similarly, regulation of oven temperature is important in baking. When we feel ill, we measure our body temperature to see if we have a fever.
To find out how hot or cold an object really is, a suitable concept is 'temperature'. In our day-to-day life, we come across a number of objects out of which some are hot and some are cold. We often decide which object is hotter than the other by touching the objects. The idea of temperature is natural to human beings as one of the basic senses. The sense organs in the human skin act as a crude thermometer. Although our subjective sensations of hot and cold are related to temperature, they can easily mislead.
2.0Temperature and heat
T The temperature is a measure of the hotness or coldness of an object. Unit of temperature : S.I. unit of temperature is Kelvin (K). Some other popular units are Celsius and Fahrenheit. Thermometers are devices used to measure the temperature of an object or a system. Thermal equilibrium Suppose two objects or systems are allowed to exchange energy. The net flow of energy is always from the object at the higher temperature to the object at the lower temperature. As energy flows, the temperatures of the two objects approach one another. When the temperatures are the same, there is no longer any net flow of energy, the objects are now said to be in thermal equilibrium. Two objects are said to be in thermal equilibrium with each other if they are at the same temperature.
Both at Room temperature
[A]

[B]

When water at is placed in a room, it absorbs heat from the surrounding if the surrounding temperature is more than and is said to be in thermal equilibrium with the surrounding.
Examples:
(i) Ice Melting : When you place an ice cube in a room, it will absorb heat from the surroundings and melt until it reaches thermal equilibrium with the room temperature.
(ii) Thermostat in a room : A thermostat regulates the temperature in a room by turning on or off a heating or cooling system. When the desired temperature is reached, the room and the thermostat are in thermal equilibrium.
Heat
The energy that flows between two objects or systems due to a temperature difference between them is called heat. In other words 'heat is the form of energy which flows between two objects or systems as a result of temperature difference between them'. Heat is also called thermal energy.
- Energy is the capacity or ability to do work
- There are various types of energy : Mechanical energy, Light energy, Heat Energy, Sound energy, Electrical energy, Chemical energy etc.
3.0Temperature scales
Thermometers measure temperature by exploiting some property of matter that is temperature-dependent. The familiar liquid-in-glass thermometer relies on thermal expansion, the mercury or alcohol expands as its temperature rises (or contracts as its temperature drops) and we read the temperature on a calibrated scale. This scale is called temperature scale. The calibrated scale made on a thermometer is called temperature scale.
The thermometers must be calibrated on a scale using some easily reproducible phenomenon, such as the melting point of ice or the boiling point of water. The assignment of temperatures to these phenomena is arbitrary (random).
To measure temperature, two fixed points are taken on thermometers or temperature measuring device. One fixed point is the melting point of ice (or freezing point of water) called ice point (lower fixed point). The other fixed point is the boiling point of water called steam point (upper fixed point).
Several systems have been proposed and used to quantify temperature; the most widely used are the Fahrenheit, Celsius, and Kelvin scales.
- Freezing point : The freezing point is the temperature at which liquid changes into solid state.
- Melting point : The melting point is the temperature at which solid state changes into liquid state.
Fahrenheit scale
On the Fahrenheit scale, water freezes at 32 degrees and boils at 212 degrees. There are 180 Fahrenheit degrees between the freezing point and the boiling point of water.
Celsius scale
The Celsius scale divides the interval between the freezing and boiling points of water into 100 degrees (instead of 180 ). Water freezes at and boils at . Most scientists and engineers use Celsius because 0 and 100 are easier to work with than 32 and 212.
Kelvin scale
The Kelvin scale divides the interval between the freezing and boiling points of water into 100 divisions. Water freezes at 273 K and boils at 373 K . This scale is based on the existence of absolute zero, the minimum possible temperature.
Absolute zero is the lowest temperature possible in the universe. At absolute zero, there is no heat and the motion of particles (atoms or molecules) ceases (stops). You can think of absolute zero as the temperature where molecules are completely frozen, with no motion. Absolute zero occurs at or .
Relation between different temperature scales
The relation between the Celsius (C), Fahrenheit (F), and Kelvin (K) scales are Where, T is temperature of any scale, L.F.P. is lower fixed point, U.F.P. is upper fixed point. or

Relation between Celsius and Fahrenheit scales
or or or or or
Relation between Celsius and Kelvin scales
or or or
4.0Numerical Ability: Solved Examples
- Example 1: The human body average temperature is . What is it in degree Celsius?
Hint: To convert into or into we must use relation between Celsius and Fahrenheit scales
Apply the formula
Solution: Given, ; ?
- Example 2: A boy suffering from the flu has a fever; his body temperature is . What is his temperature in ?
Solution: Given, ?
- Example 3: At which temperature the Celsius and the Fahrenheit scales are equal?
Solution: Let Celsius and Fahrenheit readings are same at or ) i.e.,
Now, or
or
or or
or
or
This means, is equal to .
- Example 4: Change to Kelvin.
Solution To convert into K or K into we must use relation between celsius and kelvin scales. Apply the formula C = K - 273 K = C + 273 Given, ?
5.0Measurement of temperatures
As we discussed that temperature could be measured in a simple way by using our hand to sense the hotness or coldness of an object. However, the range of temperature that your hand can withstand is too small, and our hand is not precise enough to measure temperature adequately. Therefore, other methods are used for measuring temperature.
Certain properties of matter vary with their temperature. For example, when objects are heated, they give off light of different colours. When an object is heated, it first gives off red light. As it is heated more, it appears white. Thus, colour of light emitted by a hot object gives us an idea of its temperature.
Colours of steel is different at different temperatures
But this works only for high temperatures and it is used in the production of metal alloys. The temperature of hot molten metals is determined in this way. The change in steel colour with the increase in temperature is shown in figure.
Thermometers
Instruments that measure temperature are called thermometers. When a thermometer is in contact with a system, energy is exchanged until the thermometer and the system are in thermal equilibrium with each other. One common thermometer called liquid-in-glass thermometer consists of a mass of liquid usually mercury or alcohol that expands into a glass capillary tube when its temperature rises and contracts when temperature falls.
In this case the physical property that changes is the volume of a liquid. When the crosssectional area of the capillary tube is constant, the change in volume of the liquid varies linearly with its length along the tube. We can then define a temperature in terms of the length of the liquid column.
Liquid-in-glass thermometer
A liquid-in-glass thermometer consists of following parts : (1) Glass tube : A thermometer is a sealed glass tube marked with a scale on the outside. (2) Capillary tube : Inside the glass tube there is an another thinner tube, called the capillary tube. (3) Bulb : The capillary tube ends in a bulb made of thick glass. The bulb contains the thermometric liquid, alcohol or mercury.
Selection of thermometric liquid
Alcohol is used for study of atmosphere and weather conditions. This is because alcohol remains a liquid between and . Therefore, temperatures as low as can be measured. At such temperatures, mercury would freeze as freezing point of mercury is . For common use, mercury is preferred to alcohol for the following reasons: (1) Mercury does not freeze or vaporise easily, i.e., it remains a liquid over a large temperature range. Mercury freezes at and boils at . (2) Since mercury is a good conductor of heat, it quickly attains the temperature of the body with which it is in contact also it has high density than water. (3) Mercury does not wet the surfaces in contact thus it does not stick to the inner surface of capillary tube. (4) Mercury is an opaque liquid with shining appearance like silver. Thus, it can be easily observed through the glass.
The laboratory thermometer
The thermometers available in the laboratory consists of a long sealed glass tube (also called stem) marked with a scale on the outside (see figure). Inside the glass tube there is an another thin tube, called the capillary tube (or bore) at the end of which there is a bulb made of thick glass. The bulb is filled with a liquid at the other end. The most commonly used liquid is mercury. A shining thread of mercury can be seen from outside the thermometer. As the temperature rises, the mercury in the bulb expands and rises into the capillary tube. The scale on the glass tube is marked in or . The height of the liquid in the stem gives the reading of temperature. Laboratory thermometers with various ranges are available. The commonly used laboratory thermometers have a range from to . While measuring temperature with a laboratory thermometer, the following precautions should be taken : (1) The thermometer is delicate and should be handled with care to avoid breakage.

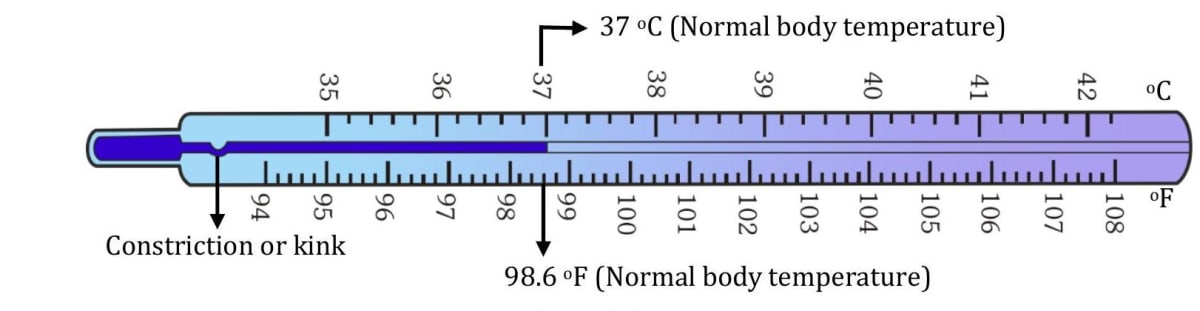
The clinical thermometer
It is a thermometer used to measure body temperature. The liquid used in it is mercury. It is used for the temperature range to . (or to ). This is because the body temperature usually does not fall below or rise above . The bigger marks normally read . If there are 5 divisions between bigger marks, each small division reads of a degree or .
- Normal body temperature is or .
Features of clinical thermometer
(1) The capillary tube has a constriction or kink (i.e., a narrower curved part) just above the bulb (see figure). It allows the mercury to rise but stops it from falling. This is useful while taking a patients temperature. The mercury level stays in position while the temperature is being noted. The thermometer has to be shaken or given jerk to bring down the mercury (2) The outer glass is made triangular or curved so that the thread of mercury is magnified and can be seen easily. (3) The bulb is made of thick glass. This is done to make the bulb strong so that it does not break under the weight of mercury or when it is shaken. You must remember that mercury is a very dense liquid. (4) To check the body temperature, a thermometer is placed in your mouth below the tongue or in your armpit for minutes. When the thermometer is taken out of your mouth, the liquid in the bulb contracts and the mercury column breaks at the kink. Therefore, the level of mercury in the stem remains the same.
Is it convenient to use laboratory thermometer to measure our body temperature?
- The temperature recorded in a laboratory thermometer falls as soon as it is taken out of the system or body whose temperature is to be measured. Thus, while measuring the body temperature by a laboratory thermometer, the temperature recorded will fall as soon as it is taken out of our mouth. Thus, we will not get the correct reading of our body temperature. Thus, it is not convenient to use laboratory thermometer to measure our body temperature.
Precautions to be observed while reading a clinical thermometer
(1)Thermometer should be washed before and after use, preferably with an antiseptic solution. (2) Ensure that before use, the mercury level is below . (3) Read the thermometer keeping the level of mercury along the line of sight. (4) Handle the thermometer with care. If it hits against some hard object, it can break. (5) Don't hold the thermometer by the bulb while reading it.
Maximum and minimum thermometer
The maximum and minimum temperatures of any day, reported in weather reports, are measured by a thermometer called the maximum - minimum thermometer. It is a combination of two thermometers:
- Maximum thermometer is a mercury-in-glass thermometer and measures the highest temperature reached in a day.
- Minimum thermometer is a alcohol-in-glass thermometer and measures the lowest temperature attained in a day.
6.0Transfer of heat
When heat is transferred, it always moves from warmer to cooler objects. The warmer object loses heat energy and becomes cooler as the cooler object gains heat energy and becomes warmer. This process of heat transfer can occur in three ways-by conduction, radiation, or convection.
Conduction
When you eat hot pizza, you experience conduction. As the hot pizza touches your mouth, heat moves from the pizza to your mouth. Conduction is the transfer of heat by the direct contact of particles of matter without the actual motion of the particles.
Heat conductors
A conductor is any material that easily transfers heat. Metals are good conductors of heat. Metals have some electrons that are free to move from one place to another within the material. These free electrons help to transfer heat energy. Gold and copper are among the best conductors of heat.
A metal block feels colder to touch than a wooden block, even though the two blocks may have the same temperature. This is because metals conduct heat energy more rapidly than a wooden block.
Heat insulators
An insulator is a material in which heat doesn't flow easily. Most pans have handles that are made from insulators. Liquids and gases are usually better insulators than solids. Air is a good insulator, and many insulating materials contain air spaces that reduce the transfer of heat by conduction within the material. Wood, plastics, wool, cork, bake lite etc. are insulator or poor conductors of heat.
- Iron is the conductor through which heat can easily flow.
- Plastic is the insulator through which heat cannot flow.
Why are cooking pans usually made of metal while their handles are made of plastics?
- If you're cooking food, you want the pan to conduct heat easily from the stove to your food, but you do not want the heat to move easily to the handle of the pan. If the heat move easily to the handle, skin of your hand may get burnt. Thus, the material of pan should be a conductor while its handle from where you hold the pan should be insulator. For this reason cooking pans are made of metals while their handles are made of plastics.
Example of conduction
Ironing clothes, frying vegetables in a pain. Touching a hot seat belt when you get into the car. Walking barefoot on hot sand. The heat from a stove top transferring into a metal pot of water.
Convection
Have you ever watched water boil in a pot? Bubbles form on the bottom and rise to the top. Hot water near the bottom of the pan circulates up, forcing cooler water near the surface to sink. This circulation carries heat through the water (see figure). This heat transfer process is called convection.
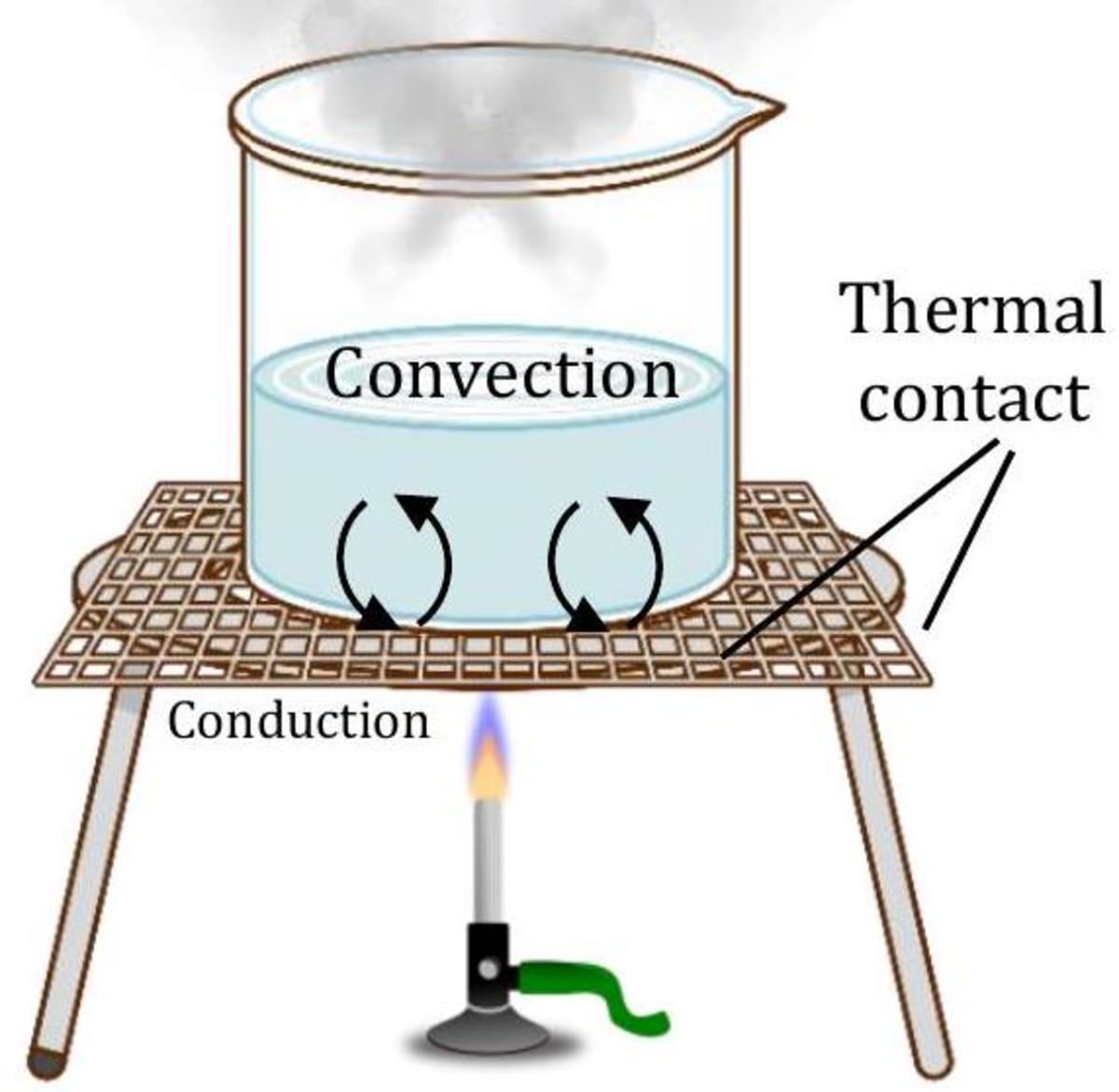
The hot water at the bottom of the pot rises to the top and replaces the cold water. Convection is the transfer of heat through the actual motion of matter such as air and water. The circular movement of currents that is set up in liquids and gases is called convection currents. Convection can be of two types: (i) natural convection (ii) forced convection
Natural convection
Natural convection is a mode of heat transfer in which the fluid motion is not generated by any external source like a pump or a fan. The heat flows only due to difference of densities within the fluid that occurs because of temperature differences in the different regions of the fluid. In natural convection, fluid surrounding a heat source receives heat, becomes less dense and rises. The surrounding, cooler fluid then moves to replace it. This cooler fluid is then heated and the process continues, forming a convection current. Natural convection occurs when a warmer, less dense fluid is pushed away by a cooler, denser fluid.
Some examples of natural convection
Sea breeze
Imagine the shore of a sea. During the day, the water is cooler than the land. Air above the warm land is heated by conduction. When the air gets hotter, its particles move faster and get farther from each other, making the air less dense. The cooler, denser air from over the lake flows in over the land [see figure (a)], pushing the less dense air upward. You feel this movement of incoming cool air as wind. The cooler air then is heated by the land and also begins to rise. The air from the sea is called the sea breeze.
Land breeze
Imagine the shore of a sea. During the night, the water is hotter than the land. Air above the warm water is heated by conduction. When the air gets hotter, its particles move faster and get farther from each other, making the air less dense. The cooler, denser air from over the land flows in over the water [see figure (b)], pushing the less dense air upward. You feel this movement of outgoing cool air as wind. The cooler air then is heated by the water and also begins to rise. The air from the land is called the land breeze.


Sea breeze and land breeze
Land and sea breezes are caused by the uneven heating of land and water, during the day.
How does the heat travel in air? In which direction does the smoke go? Try an activity to show it.
- The air near the heat source gets hot and rises. The air from the sides comes in to take its place. In this way the air gets heated. The following activity confirms this idea :
Light a candle. Keep one hand above the flame and one hand on the side of the flame (see figure). Be careful. Keep your hands at a safe distance from the flame so that they do not get burnt. The hand above the flame feels hot. This means that hot air moves in upward direction and towards the top, the air gets heated by convection. On the sides, however, there is no convection and air does not feel as hot as at the top.

Forced convection
Forced convection is a mode of heat transfer in which fluid motion is generated by an external source like a pump, fan, blower, etc. It is one of the main methods of transferring heat efficiently.
Examples of forced convection Forced convection is found very common in everyday life. It includes following applications: (1) Central heating systems. (2) Central cooling systems, air conditioning. (3) Electric heat convectors or blowers used for room heating at home. (4) Heat exchanger like condensers, heaters, coolers, etc. used in industries. (5) Car, truck or bus engines are cooled by convection currents in the water pipes.
7.0Radiation
On a beautiful, clear day, you walk outside and notice the warmth of the Sun. You know that the Sun heats Earth, but how does this transfer of heat occur? It cannot reach us by conduction or convection because almost no matter exists between the Sun and Earth. Instead, heat is transferred from the Sun to Earth by an another process called radiation. Radiation is the process of heat transfer in which no material medium is required. Heat transfer by radiation occurs when energy is transferred in the form of waves. The waves which mainly carry heat from Sun to Earth are infrared rays. These waves are also called heat waves or thermal radiation. Heat waves are just like light but unlike light they are invisible.
All objects emit and absorb radiation
The transfer of heat energy by radiation can occur in empty space, as well as in solids, liquids, and gases. The Sun is not the only source of radiation. All objects emit heat waves, although warm objects emit more radiation than cool objects. Some common examples of radiation are : (1) The warmth you feel when you sit next to a fireplace is due to heat transferred by radiation from the fire to your skin. (2) When we sit in front of a room heater, we get heat by radiation. (3) A hot utensil kept away from the flame cools down as it transfers heat to the surroundings by radiation. (4) Our body too, gives heat to the surroundings and receives heat from it by radiation.
Some surfaces absorb more energy than others
Black surfaces absorb almost all the thermal radiation that falls on them. For example, black coal tar road gets very hot in the summer because it effectively absorbs thermal radiation. A silver mirror surface reflects most thermal radiation, absorbing very little.
Why is it more comfortable to wear white or light - coloured clothes in the summer and dark-coloured clothes in the winter?
- Light coloured clothes reflect most of the heat that falls on them and, therefore, we feel more comfortable wearing them in the summer. Dark surfaces absorb more heat and, therefore, we feel comfortable with dark coloured clothes in the winter.
Woolen clothes keep us warm in winter
In the winter, we use woolen clothes. Wool is a poor conductor of heat i.e., it is a heat insulator. Moreover, there is air trapped in between the wool fibers. Since air is also a heat insulator, it prevents the flow of heat from our body to the cold surroundings. So, we feel warm.
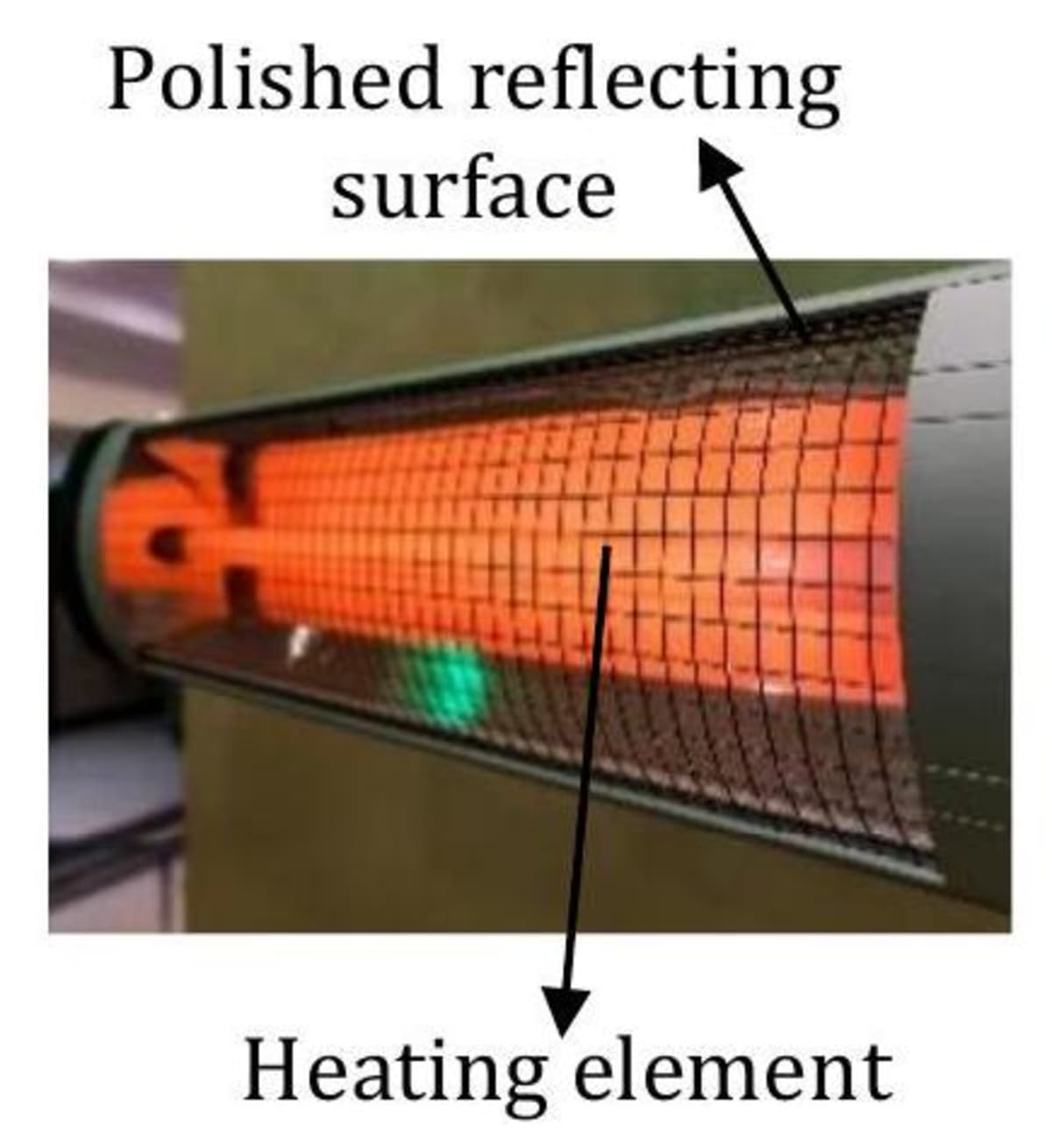
Electric room heater
Electric room heaters are provided with a polished metal surface behind the heating element (see figure). This surface reflects almost all the radiant heat from the heating element that falls on it. This makes the room heater more effective.
Electric heater works on the principal of "Heating effect of electric current".
Heat conduction cannot occur through a vacuum
Conduction happens only if there are particles available to collide with one another. Conduction does not occur in the vacuum of space. One way to create an excellent heat insulator on Earth is to make a vacuum. A vacuum is empty of everything, including air. A thermos bottle keeps liquids hot for hours using a vacuum. A thermos is a container consisting of a bottle surrounded by a slightly larger bottle. Air molecules have been removed from the space between the bottles to create a vacuum.
8.0Differences between different modes of heat transfer
9.0Concept Map

10.0Some Basic Terms
- Light coloured : Colours having relatively small amount of colouring agent.
- Lukewarm : Used about liquid which are slightly warm.
- Thermal expansion : Tendency of matter to change its volume in response to temperature change.
- Vacuum: A space that is completely empty of all substances including air or other gases.
- Freezing point : The temperature at which a liquid turns into solid when cooled.
- Boiling point : Temperature at which liquid converts into steam or vapour.
- Molecules : When two or more atoms are combined together.
- Metal alloys : A substance that combines more than one metal or mixes a metal with other non-metallic elements.
- Mass : The amount of matter in a particle or object.
- Density: The mass of an object contained per unit volume.
- Antiseptic solutions : Antiseptic solutions are the drugs that are applied to living tissues to kill the bacteria.
- Fluid : Substance that can flow.
- Denser: When particles are closely packed together.
- Mean position : The bodies position when there is no net force acting on it.
- Infrared : It is an electromagnetic radiation which is invisible to the human eye but can be felt has heat.
11.0SOLVED EXAMPLES
1. Convert the boiling temperature of gold 2966, into degree Fahrenheit and Kelvin.
Solution:
To convert boiling temperature of gold from Celsius to Fahrenheit, we use the formula. For Kelvin we can use the formula
2. Convert the temperature of the coldest area in a freezer, -10, to degrees Celsius and Kelvin.
Solution:
To convert the temperature of Fahrenheit we can use the formula. For Kelvin we can use,
3. Convert the temperature of hot water, 54 into degree Fahrenheit and Kelvin.
Solution:
To convert the temperature from Celsius to Fahrenheit we can use the formula. For Kelvin we can use the formula
4. Convert 223 to Kelvin
Solution:
To convert degree Fahrenheit and Kelvin, we can use the formula
5. Convert 425 K to Celsius
Solution:
To convert Kelvin to Celsius we can use the formula,
Related Article:-
Join ALLEN!
(Session 2026 - 27)