Physical and Chemical Changes
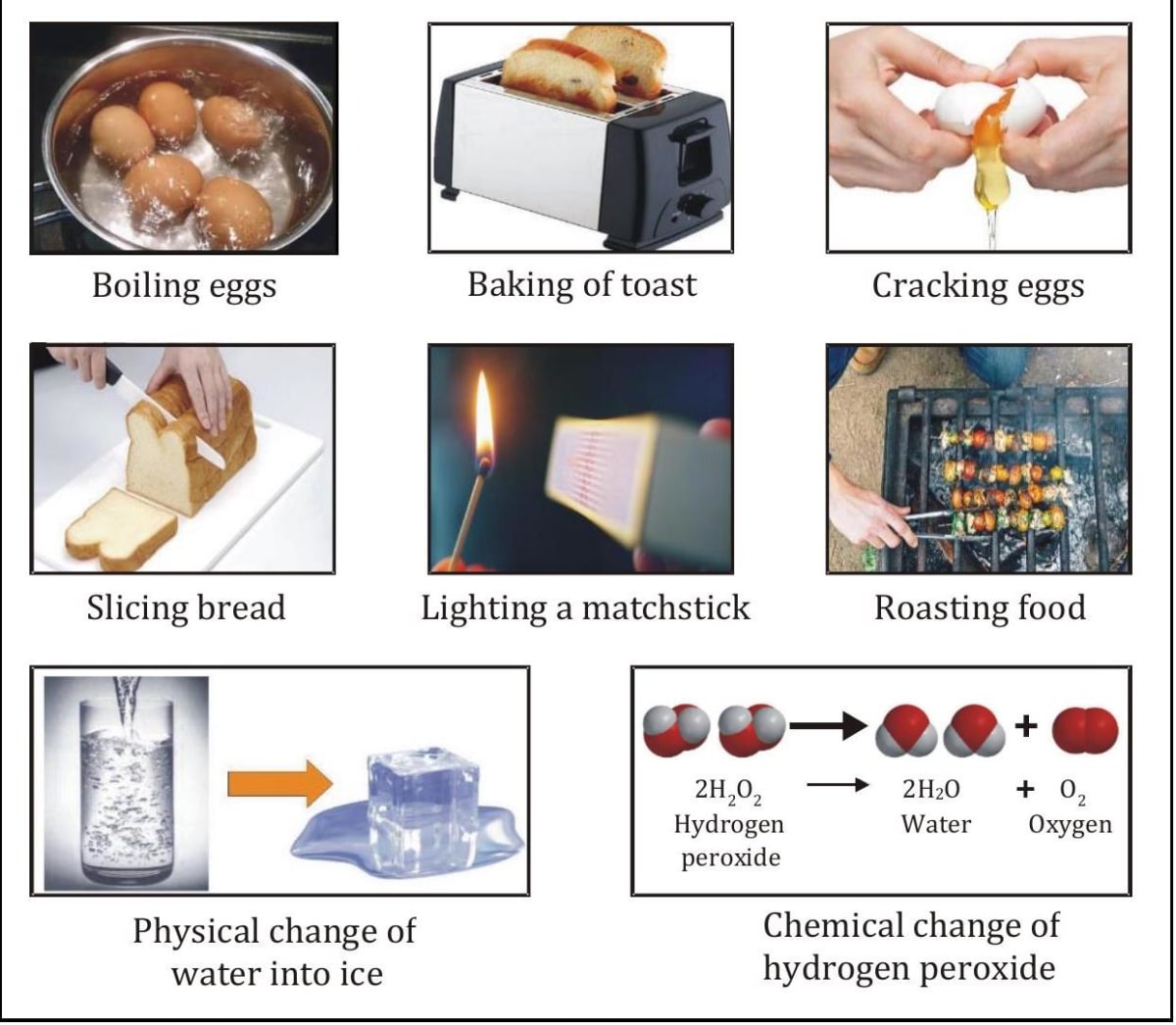
- When their is change in certain properties like change in colour, shape, size, composition, position, etc we, say that change has taken place.
- There are different type of change like slow change and fast change, reversible and irreversible change, natural and man-made change, desirable and undesirable change, etc.
1.0Introduction
We know that nothing on this Earth ever really disappears. It appears like snow disappears in the Sun or the flames, that wood vanishes in a fire, but that is not actually happening. Snow melts and becomes liquid which on evaporation turns into gas that rises into the atmosphere similarly burnt wood turns into ash and produces smoke. Every day, you experience changes in matter. Cooking eggs, burning leaves, freezing water and mixing oil and vinegar to make salad dressing, involve changes in matter.
Matter can never be created or destroyed. It just changes its form. All matter is made up of tiny molecules and when these molecules are changed or moved around, the matter changes its form. When our mother asks us to dissolve sugar in water to make sugar solution, this is also a change.
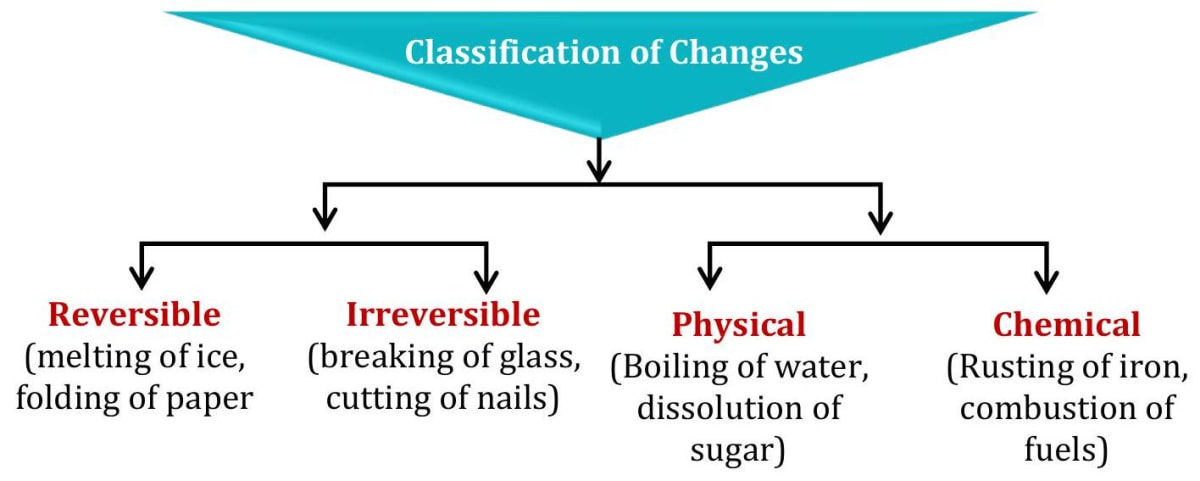
We classify these changes into two categories (i) Physical changes (ii) Chemical changes
2.0Physical changes
(i) Cut the sheet of paper into four square pieces. Cut one square into four square pieces. Lay these pieces on the table so that the pieces acquire the shape of the original piece of paper. (ii) Take some ice cubes in a glass tumbler and melt a small portion of ice by placing the tumbler in the Sun. You have now a mixture of ice and water. Now place the tumbler in the freezing chamber of your fridge for a few hours. (iii) Watch the bulb of your table lamp carefully. Watch the filament becoming white hot and emitting light when you switch on the lamp. Switch it off and observe. (iv) Take some boiling water and place an inverted pan over the rising steam at some distance and observe.
Observation
(i) The pieces of paper cannot be joined back to make the original but the property of the paper remains the same. (ii) The melted ice forms water and water freezes back to form ice. (iii) The bulb is back to its original state and does not give light. (iv) Some water droplets are found to condense on the inner surface of the pan.
Conclusion
(i) The paper undergoes a change in shape and size. (ii) Ice undergoes a change in state. (iii) Bulb undergoes a change in colour. (iv) Steam undergoes a change in state.
In all the above cases, no new substance is formed and all changes are reversible and temporary. Hence, all of them undergo a physical change. Properties such as shape, size, colour and state of a substance are called its physical properties. A change in which a substance undergoes a change in its physical properties is called a physical change. A physical change is generally reversible. In such a change, no new substance is formed.
Characteristic features of physical changes
(1) The identity of the substance is maintained. (2) The change is temporary. (3) Heat change may or may not take place. (4) Only the physical state or some of the physical properties of the substances are changed. In a physical change, the substance that is involved remains the same, even though its form or state may change. A piece of wood when cut into pieces, is still a wood. When ice melts, it is still the same substance. Changes of state-melting, freezing, evaporation, condensation and sublimation, are physical changes.

The most important characteristics of physical change is that there is no new substance is formed in physical change.
Examples
Some more examples of physical changes are (i) Mixing salt and water. (ii) The falling of any object under gravity. (iii) Vase breaks into pieces. (iv) Ink being absorbed by a blotting paper or chalk. (v) Stretching of a rubber band. (vi) Grinding a fruit to pulp in a mixer. (vii) Dew drops condensing on a leaf at dawn. (viii) Copper metal being drawn into wires. (ix) Blowing up a balloon. (x) Shaping clay into different shapes.
In a physical change, the particles of a substance may move closer or farther apart or they may mix with particles of other substances. However, no new kind of particles are produced.
3.0Reversible and irreversible changes
Changes that can be reversed are called reversible changes. Physical changes are often reversible, but not always. You can reverse the physical change that occurs when you melt ice by cooling the water until it freezes again. You cannot reverse the physical change that occurs when wood is sawed into pieces. Changes that cannot be reversed are called irreversible changes.
- Mostly physical changes are reversible in nature but some physical change are irreversible in nature like breaking of glass, tearing of paper, cutting of wood, etc.
Some more examples of reversible changes are: (i) Stretching of a rubber band. (ii) Blowing up a balloon. (iii) Shaping clay into different shapes.
Some more examples of irreversible changes are: (i) Rusting of iron. (ii) Mixing an acid with a base, producing water and salt. (iii) Cooking examples: popcorn, cake, pancakes and eggs. (vi) Frying an egg. (vii) Lighting a matchstick.
4.0Chemical changes
Moisten the nails with water. Place all the above substances in the corner of your house for two days and then check them individually and observe.
Observation
(i) Iron nails get covered with a reddish-brown substance which is different from iron is formed. (ii) The potato gets almost black and gives a foul smell. (iii) Cooked dal or vegetable gets discoloured and gives a foul smell. (iv) The milk curdles and gives foul smell.
Conclusion
(i) There is a formation of reddish-brown substance which is known as rust, which has completely new properties than iron nail. (ii) The potato gets discoloured when exposed to air, hence it becomes dark and gives foul smell. (iii) When exposed to air, oil present in cooked dal or vegetables spoiled, which in result discoloured the food and gives foul smell. (iv) Due to the prolonged exposure to air milk ferments and gives foul smell.
Can you reverse these changes? Obviously, it is not possible. In all these changes, state as well as composition of the substance change. New substances are formed. The changes are irreversible and the substances may change their colour, smell or taste. Such a change is called a chemical change. Chemical changes occur when two or more substances combine and react with each other and produces new substances. In a chemical change, matter doesn't just change its form, as it does in a physical reaction. Chemical changes cause the molecules of matter to change. A change in which one or more new substances are formed is called a chemical change. A chemical change is also called a chemical reaction. This is more than a change in shape or state. Most of the time, an entirely new kind of matter is created. Baking is a perfect example of a chemical change. Imagine all of the ingredients needed to make a batter of brownies. Eggs, flour, oil, water and cocoa are stirred together. After heating the mixture to a high temperature for a set period of time, you have something very different than the parts you put into the bowl. Burning a piece of paper is also a chemical change. The basic substance of the paper is changed into something new, smoke and ashes. These new substances have very different molecules than the original piece of paper.
Characteristic features of chemical changes
(1) The identity of original substance is completely lost. (2) The change is permanent. (3) The change is generally accompanied by energy change. (4) The change cannot be reversed.
- The most important characteristics of chemical change is that in chemical change always new substance is formed.
Some more examples of chemical changes are
(i) Rusting of iron. (ii) Mixing an acid with a base, producing water and salt. (iii) Photosynthesis - a process in which carbon dioxide and water are changed into sugars by plants. (iv) Digestion of food. (v) Cooking examples: popcorn, cake, pancakes and eggs. (vi) Frying an egg. (vii) Lighting a matchstick. (viii) Mixing of chemicals. (ix) Rotting of fruit. (x) Explosion of fireworks.
5.0Identifying changes
It is difficult to see the chemical change in wax by looking at a burning candle. We can often see the results of a chemical change; however, we can see the light from a candle and the colour and firmness of a cooked egg. So, how can we tell if a chemical change has occurred?
In order to tell the difference between a chemical change and a physical change, we need to observe some evidences.


6.0Chemical reactions and equations
Chemists have learned that a chemical change always involves a rearrangement of the ways in which the atoms are grouped. For example, when the methane in natural gas combines with oxygen then, carbon dioxide and water are formed. Such a chemical change is called as a chemical reaction.
Chemical equation
When chemicals react or when a chemical substance breaks up, new substances are formed. These changes are shown with the help of the symbols and formula represents a chemical equation. A chemical equation is the shorthand notation used to represent a chemical reaction. We represent a chemical reaction by writing a chemical equation, in which the chemicals present before the reaction, the reactants are shown to the left of an arrow and the chemicals formed by the reaction, the products, are shown to the right of an arrow.
Reactants: The substance / substances, which take(s) part in a chemical reaction.
Products: The new substance / substances formed as a result of a chemical reaction are called products. The arrow indicates the direction of the change and is read as "yields" or "produces".
Reactants Products
A balanced chemical equation verifies law of conservation of mass. The law of conservation of mass was given by Antoine L. Lavoisier.
According to "the law of conservation of mass"
For the reaction of methane with oxygen, we have
Note: - From this equation, it has been concluded that the products contain the same atoms as the reactants but that the atoms are associated in different ways. That is, a chemical reaction involves changing the ways the atoms are grouped. In a chemical reaction, atoms are neither created nor destroyed. All atoms present in the reactants must be accounted among the products. In other words, there must be the same number of each type of atom on the product side as on the reactant side of the arrow. To make sure that the equation for a reaction obeys this rule, balancing the chemical equation is done.
7.0Identifying Reactions
Characteristics of a chemical reaction:
- Heat, light or any other radiation (like, ultraviolet) may be given off or absorbed.

- Sound may be produced.

- A change in smell may take place or a new smell may be given off.

- A colour change may take place.

- A gas may be released.

8.0Rusting
When a piece of iron is left in moist air for some time, a red-brown solid is deposited over it. Iron combines with the oxygen and moisture (i.e. water vapour) of the air to form a new substance called rust-the red brown solid. The forming of rust is called rusting.
Factors which promote corrosion
For rusting the presence of both oxygen and water (or water vapour) is essential. Infact if the content of moisture in air is high, which means if it is more humid, rusting becomes faster.
Rusting of iron takes place in the presence of moisture and air, so the presence of oxygen and water vapour in air are necessary condition for rusting of iron.
Prevention of rusting
As stated earlier rusting will take place only, if iron is simultaneously acted upon by water and oxygen (from air). So, if we prevent the iron articles to come in contact with air or water or both, the rusting will stop. Rusting of iron surface exposed to the air is prevented by application of protective coatings on iron. (1) By painting: The corrosion of a metal can be prevented simply by painting the metal surface by grease or varnish that forms a protective layer on the surface of the metal which protect the metal from moisture and air. (2) Electroplating: It is a very common and effective method to check corrosion. The surface of metal is coated with chromium, nickel or aluminium etc. by electrolysis also called electroplating. They are quite resistant to the attack by both air and water and check corrosion. If the surface of metal is electroplated by zinc, it is known as galvanisation and in case tin metal is used, then the process is called tinning. ships are made of iron and a part of them remains under water. On the part above water also, water drops keep clinging to the ship's outer surface. Moreover, the water of the sea contains many salts. The salt water makes the process of rust formation faster. Therefore, ships suffer a lot of damage from rusting in spite of being painted. So much so, that a fraction of ship's iron has to be replaced every year.
9.0Crystallisation
The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation. Crystallisation is a process that separates a pure solid in the form of its crystals from a solution. It is an example of physical change.
The soluble impurities do not get removed in the process of evaporation.
Some solids decompose or get charred on heating to dryness during evaporation. But such impurities get removed in crystallisation. Crystallisation is a better technique than evaporation to dryness.
Mother liquor: A liquid which is obtained after crystallization.

Uses of crystallisation:
Purification of salt that we get from sea water. Separation of crystals of alum (phitakari) from impure sample.
10.0Building Concepts
Dissolving sugar in water is a physical or a chemical change? Explain.
- Dissolving is a physical change. When you dissolve sugar in water, the sugar particles spread out and mix with the water particles, but they are still there. You can reverse the process by evaporating the water and collecting the sugar. Physical changes are usually caused by some form of motion or pressure or a change in temperature. (i) When water boils and turns into steam, it is undergoing a physical change caused by a change in temperature. (ii) When wool is spun into thread, the physical change is caused by a motion. (iii) A sheet of metal is the result of powerful pressure by machines that flattens the metal.
How chemical changes are important for us?
- We rely on chemical changes to survive. The clothes we wear and the food we eat are the results of chemical changes. There are millions of chemical changes going on around us. Some are even happening in our body. Plants use energy from the sun to combine water and carbon dioxide, which react to form sugar and oxygen. When we eat these plants and inhale oxygen from the air, the sugar and oxygen react in our cells to produce water, carbon dioxide and energy. From this reaction the energy generated is used for our daily activities. Ozone protects us from the harmful ultraviolet radiation which come from the sun. Ozone absorbs this radiation and breaks down to oxygen. We call the breaking down of ozone a chemical change.
11.0Basic terminology
- Physical properties - Properties such as shape, size, colour and state of a substance are called its physical properties.
- Chemical properties - The composition of the substance is the chemical properties of that substance.
- Reversible change - Changes that can be reversed are called reversible changes.
- Irreversible change - Changes that cannot be reversed are called irreversible changes.
- Magnesium Ribbon - Ribbon is formed by magnesium metal that burns with a dazzling white light.
- Copper sulphate - Copper sulphate is salt obtain when copper oxide reacts with sulphuric acid.
- Carbon dioxide gas - gas turns lime water milky.
- Chemical equation - A chemical equation is the shorthand notation. Used to represent a chemical reaction.
- Rusting - Iron combine with the oxygen and moisture of air to form new substance called rust (Reddish brown layer) and the process is called rusting.
- Galvanisation - When metal is electroplated by zinc, it is known as galvanisation.
- Electroplating - The surface of metal coated with nickel, chromium, aluminium, etc. by electrolysis called.
- Tinning - When tin metal is used for coating metal surface is called tinning.
- Crystallization - Process of cooling hot concentrated solution of a substance to obtain crystal is called crystallization.
12.0Memory Map
13.0Differences between physical and chemical Changes
Physical changes
No new substance is formed
Temporary change
Chemical changes
Formation of at least one new substance
Permanent change
Chemical reaction
The process in which a substance or substances undergo a chemical change to produce new substances
Chemical equation
A chemical equation is a shorthand way of describing the events that occur in a chemical change or reaction.
Rusting of iron
Rusting is the phenomenon of the deposition of a reddish-brown coating on the surface of iron by the action of moist air, and the reddish-brown coating is known as rust.
Prevention rusting of iron
Painting, Oiling, Galvanization, Coating with chromium
Crystallization
The process of obtaining crystals of pure substances from their saturated solution is called crystallisation.
Related Article:-
Join ALLEN!
(Session 2026 - 27)