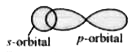
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 4: MOT and Hydrogen Bonding)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-2: CONCEPT APPLICATOR|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 2: Octet Rule, Resonance, Dipole Moment and Bond Polarity)|13 VideosCHAPTERWISE NUMERIC/INTEGER ANSWER QUESTIONS
DISHA PUBLICATION|Exercise CHAPTER 28- BIOMOLECULES|10 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 3: VSEPR Theory, VBT Theory and Hybridization)
- The angle between the overlapping of one s-orbital and one p-orbital i...
Text Solution
|
- The equilateral shape has
Text Solution
|
- Which one of the following has the shortest carbon-carbon bond length ...
Text Solution
|
- Which of the following does not have a tetrahedral structure ?
Text Solution
|
- In which one of the following molecules , the central atom said to ado...
Text Solution
|
- Which of the following two are isostructural?
Text Solution
|
- The decreasing valuse of bond angles from NH(3) (106^(@)) to SbH(3)(10...
Text Solution
|
- Among the following the pair in which the two species are not isostruc...
Text Solution
|
- Which of the following molecules has trigonal planar geometry ?
Text Solution
|
- Linear combination of two hybridised orbitals belonging to the two ato...
Text Solution
|
- Which of the following statement is not correct for sigma and pi- bond...
Text Solution
|
- How many sigma and pi bonds are present in toluene ?
Text Solution
|
- The type of bonds present in sulphuric anhydride
Text Solution
|
- How many sigma bonds are in a molecule of diethyl ether, C(2)H(5)OC(2)...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- Which molecule is planar?
Text Solution
|
- Amongst the following, the molecule/ion that is linear is:
Text Solution
|
- The trigonal bipyramidal geometry results from the hybridisation
Text Solution
|
- The true statements from the following are 1. PH(5) and BiCl(5) do n...
Text Solution
|