Recommended Questions
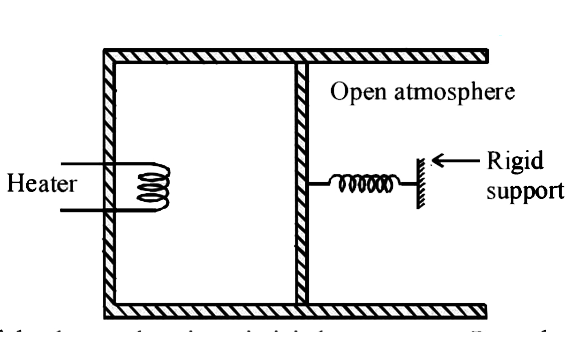
- An ideal monatomic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- An ideal monatonic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- An ideal monoatomic gas is confined in a horizontal cylinder by a spri...
Text Solution
|
- An ideal monatomic gas is confined in a cylinder by a spring-loaded pi...
Text Solution
|
- Two moles of an ideal monoatomic gas are confined within a cylinder by...
Text Solution
|
- 2 moles of a diatomic gas are enclosed in a cylinder piston arrangment...
Text Solution
|
- Two moles of an ideal monoatomic gas are confined within a cylinder by...
Text Solution
|
- एक सिलिण्डर में एक आदर्श एकपरमाणुक गैस, स्प्रिंग भारित व द्रव्यमानहीन ...
Text Solution
|
- 2.00 mole of a monatomic ideal gas (U=1.5nRT) is enclosed in an adiaba...
Text Solution
|