Text Solution
Verified by Experts
Topper's Solved these Questions
FEBRUARY 2018
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS|Exercise CHEMISTRY (SECTION -II)|24 VideosFEBRUARY 2016
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS|Exercise SECTION-I|41 VideosFEBRUARY 2019
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS|Exercise SECTION - D|21 Videos
Similar Questions
Explore conceptually related problems
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS- FEBRUARY 2018-CHEMISTRY (SECTION -II)
- Baeyer's reagent is :
Text Solution
|
- Identify 'A' in the following reaction : A+2Na overset("Dry")underse...
Text Solution
|
- An antifertility drug is :
Text Solution
|
- Write balanced chemical equations for the conversion of CrO(4)^(2-) to...
Text Solution
|
- Explain the geometry of [Co(NH(3))(6)]^(3+) on the basis of hybridisat...
Text Solution
|
- Why ethanol has higher boiling point than ethane ?
Text Solution
|
- Write only reactions for the preparation of benzophenone from benzonit...
Text Solution
|
- What is the action of p-toluene sulphonylchloride on ethylamine and di...
Text Solution
|
- What are amino acids ? Write the correct reaction for formation of pep...
Text Solution
|
- Define : (a) Antiseptics (b) Antioxidants
Text Solution
|
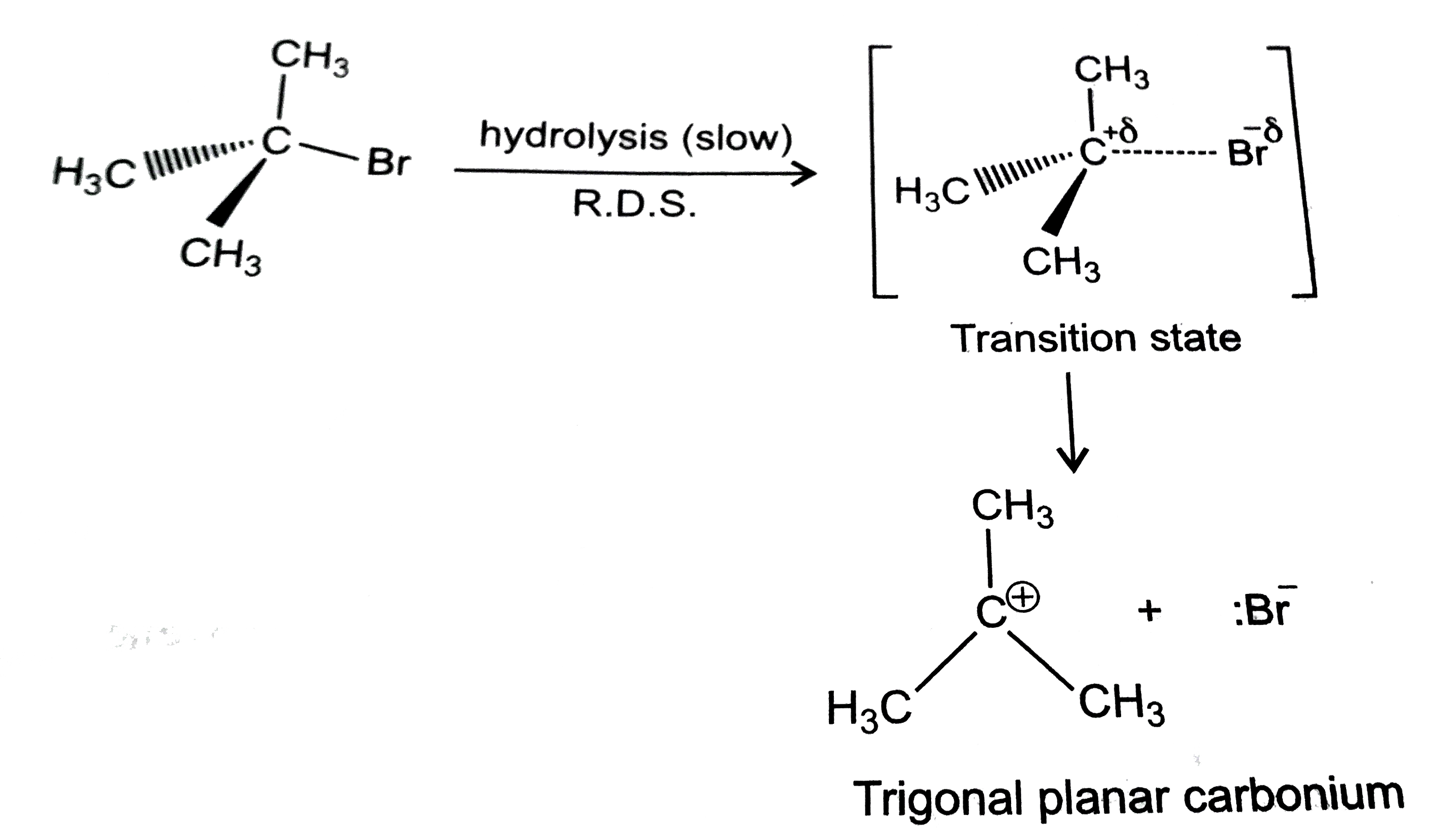
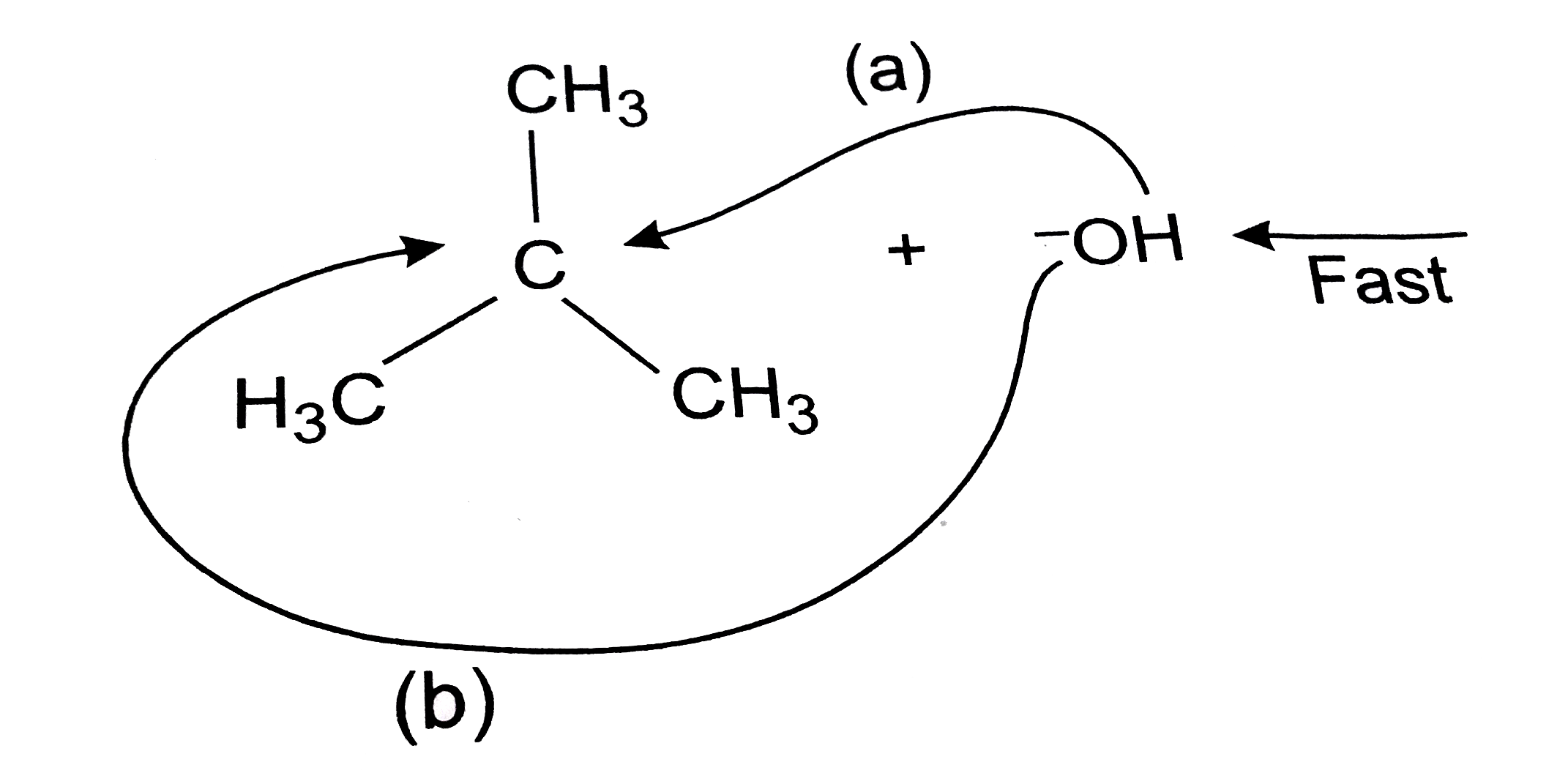
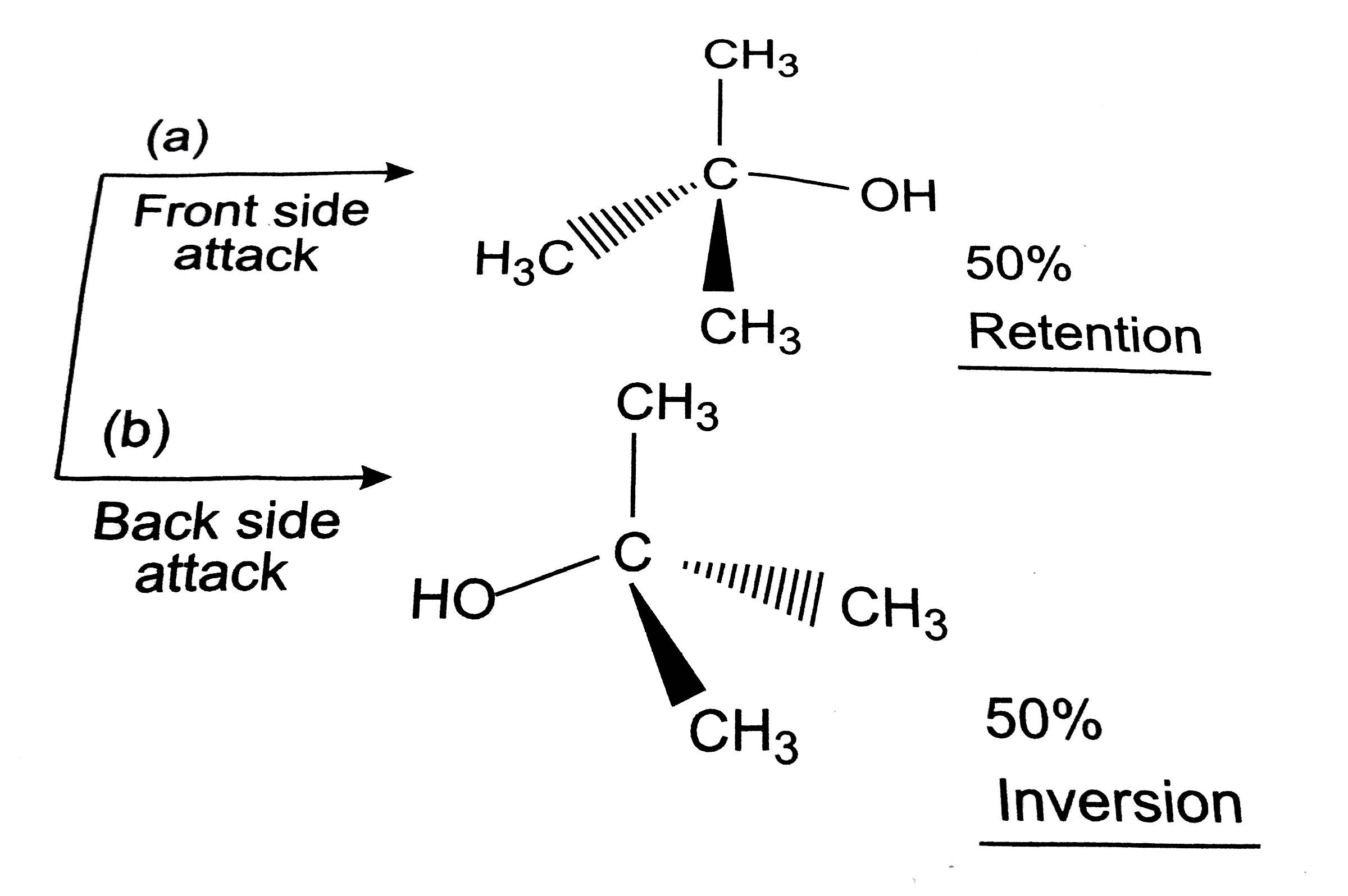
- Explain only reaction mechanism for alkaline hydrolysis of tert-butylb...
Text Solution
|
- Complete and rewrite the balanced chemical equations : Chlorobenzene...
Text Solution
|
- Complete the chemical equations : Isobutyraldehyde overset(50% KOH...
Text Solution
|
- Complete and rewrite the balanced chemical equations : Butanone + 2,...
Text Solution
|
- Prepare carbolic acid from benzene sulphonic acid. Write a chemical...
Text Solution
|
- Explain the preparation and uses of nylon-2-nylon-6.
Text Solution
|
- How glucose is prepared from cane sugar ? Write the formula of the co...
Text Solution
|
- What is lanthanide contraction ? Explain the cause of lanthanide con...
Text Solution
|
- What is the action of the following reagents on ethanoic acid ? (a) ...
Text Solution
|
- Identify 'A' and 'B' in the following reaction and rewrite the complet...
Text Solution
|