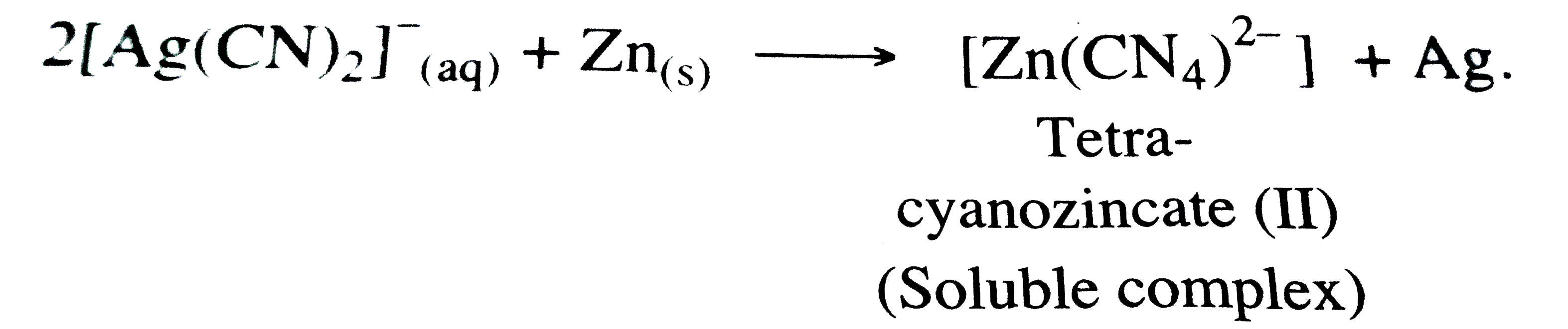(a) STEP -1 : The metal is leached with doilute solution of NaCN or KCN in the presence of air `(O_(2))` , so that a soluble complex of the metal is obtained .
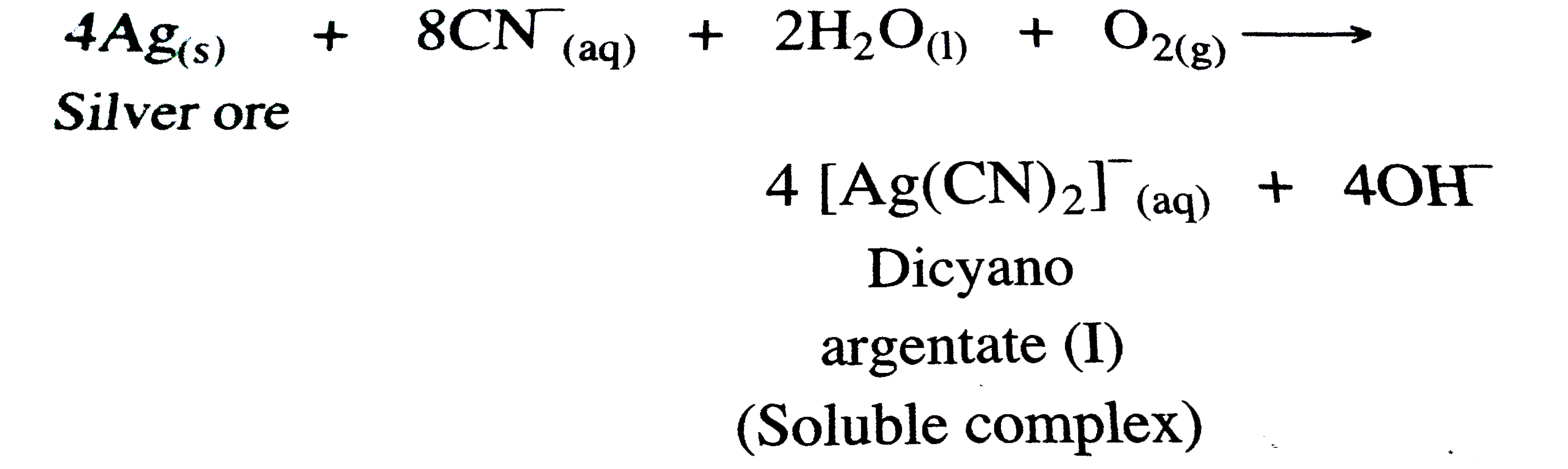
,
STEP - 2 : From tbe soluable complex `[M(CN_(2))]^(-)` , the metal Ag is precipitated by more electropositive or active metals like Zn .
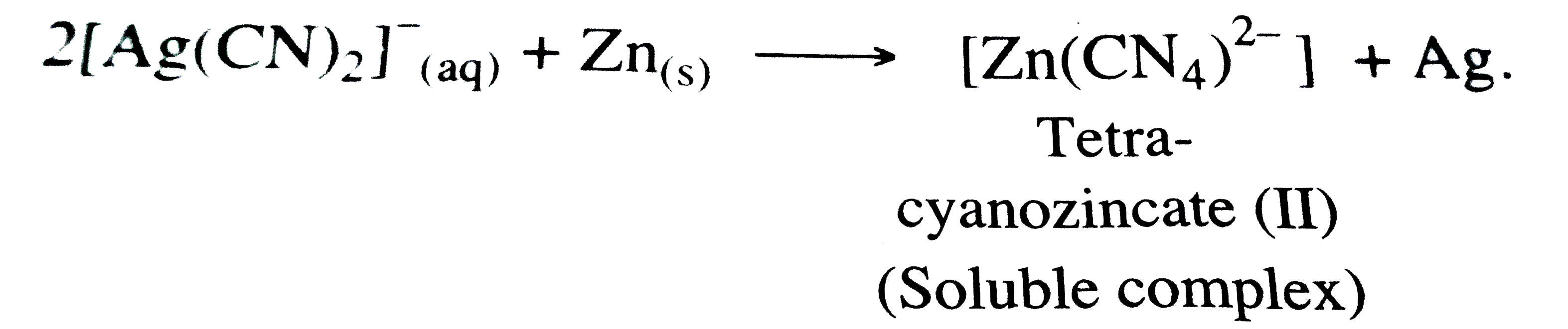
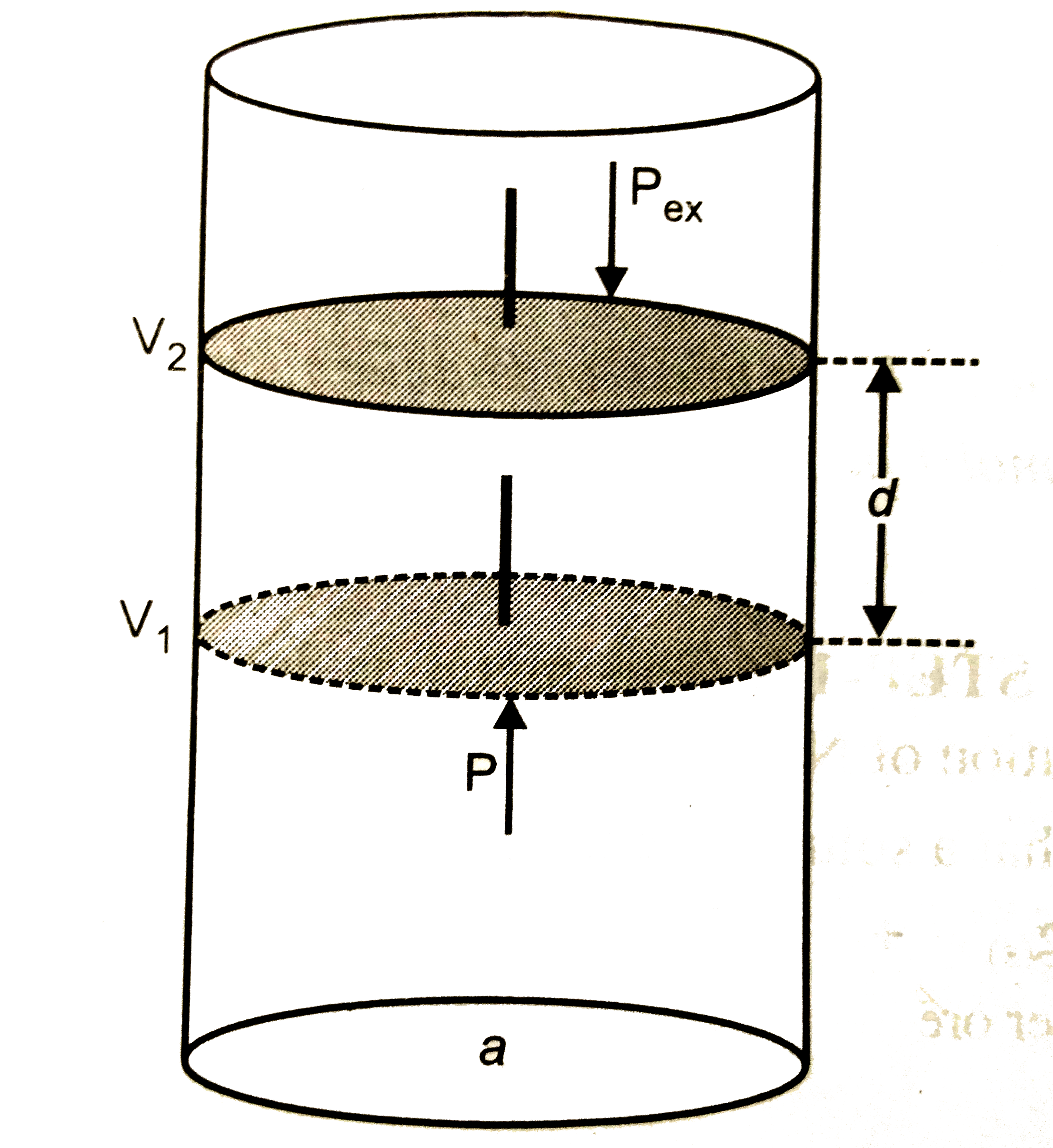
(b) Consider a certain amount of an ideal gas enclosed in an ideal cylinder fitted with ,massless , frictionless rigid movable piston at pressure P, occupying volume `V_(1)` at Temperature T .
As the gas expands , it pushes the piston upward through a distance d against sternal force F , pushing the surroundings .
The work done by the gas is,
`W = "Opposing force" xx "Distance"`
`W = -Fxxd`
-ve sign indicates the lowering of energy of the system during expansion .
If a is the - section area of the cylinder or piston , then ,
` W = -F/a xx d xx a`
Now the pressure is,
` P _("ex") = F/a`
While volume change is ,
` Delta V = d xxa`
` :. W = -P _("ex") xx Delta V`
If during the expansion , the volume changes from `V_(1) and V_(2)` then ,
` Delta V = V_(2) - V_(1)`
` :. W = -P _("ex")(V_(2)-V_(1))`
(c ) Given that :
` d = 7*86 g//cm^(3)`
` a = 288 ` ppm
or ` = 2*88 xx 10^(-8)` cm
` N_(A) = 6*022 xx10^(23) "mol"^(-1)`
Molar mass = 56 g/mol
` d = (ZxxM)/(a^(3) xxN_(A))`
` Z = (d xx a^(3) xx N_(A))/M`
` = (7*86 xx(2*88 xx10^(-8))^(3)xx6*022 xx10^(23))/56`
` = 2*01 ~~ 2`
Hence, the unit cell of crystal lattice is of bcc type.
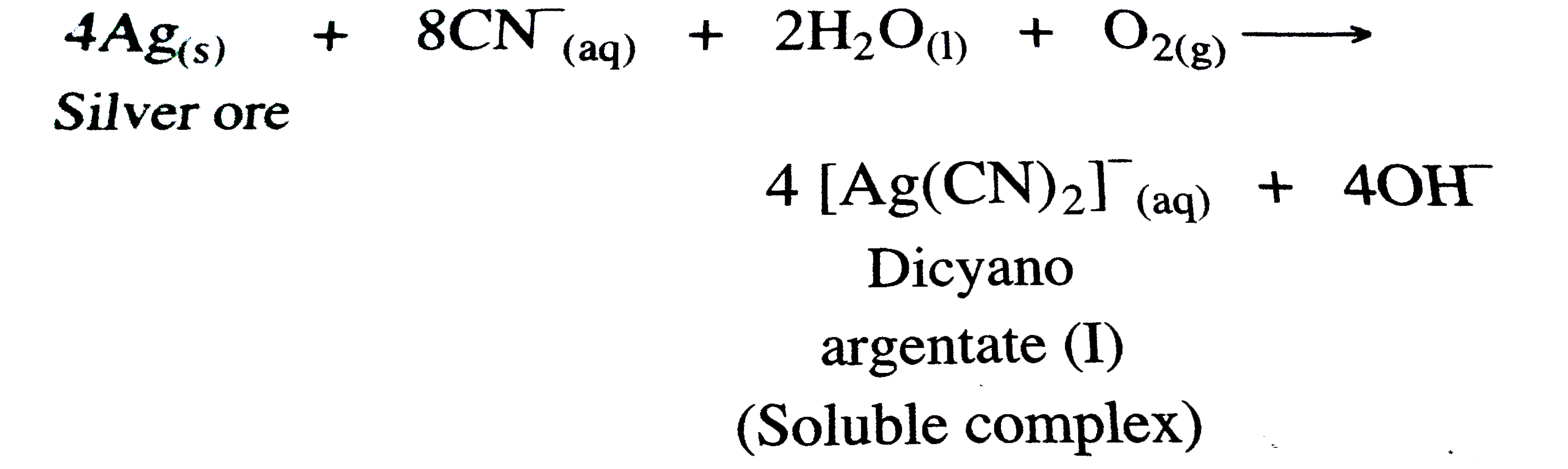 ,
,