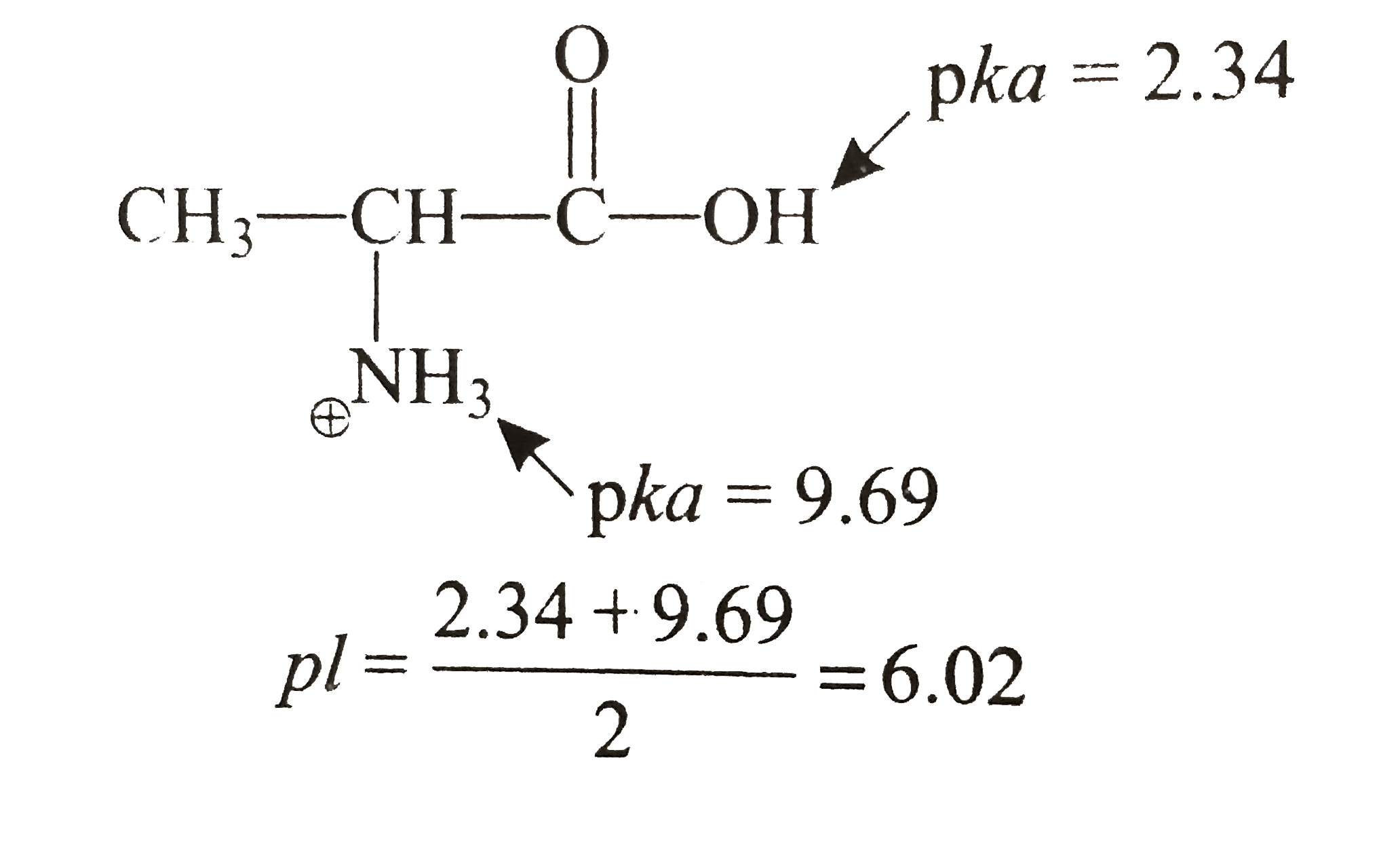Similar Questions
Explore conceptually related problems
Recommended Questions
- The isoelectric point(pl) of an amino acid is the pH of wihc it has no...
Text Solution
|
- Which of the following amino acides has an isopropyl group as its side...
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- The pKa values for the three acidic group P,Q,R are 4.3, 9.7 and 2.2 r...
Text Solution
|
- The isoelectric point(pl) of an amino acid is the pH of wihc it has no...
Text Solution
|
- The isoelectric point(pl) of an amino acid is the pH of wihc it has no...
Text Solution
|
- The isoelectric point(pl) of an amino acid is the pH of wihc it has no...
Text Solution
|
- An amino acid is characterized by two pKa values the one corresponding...
Text Solution
|
- Isoelectric point of an amino acid is the pH at which the amino acid m...
Text Solution
|