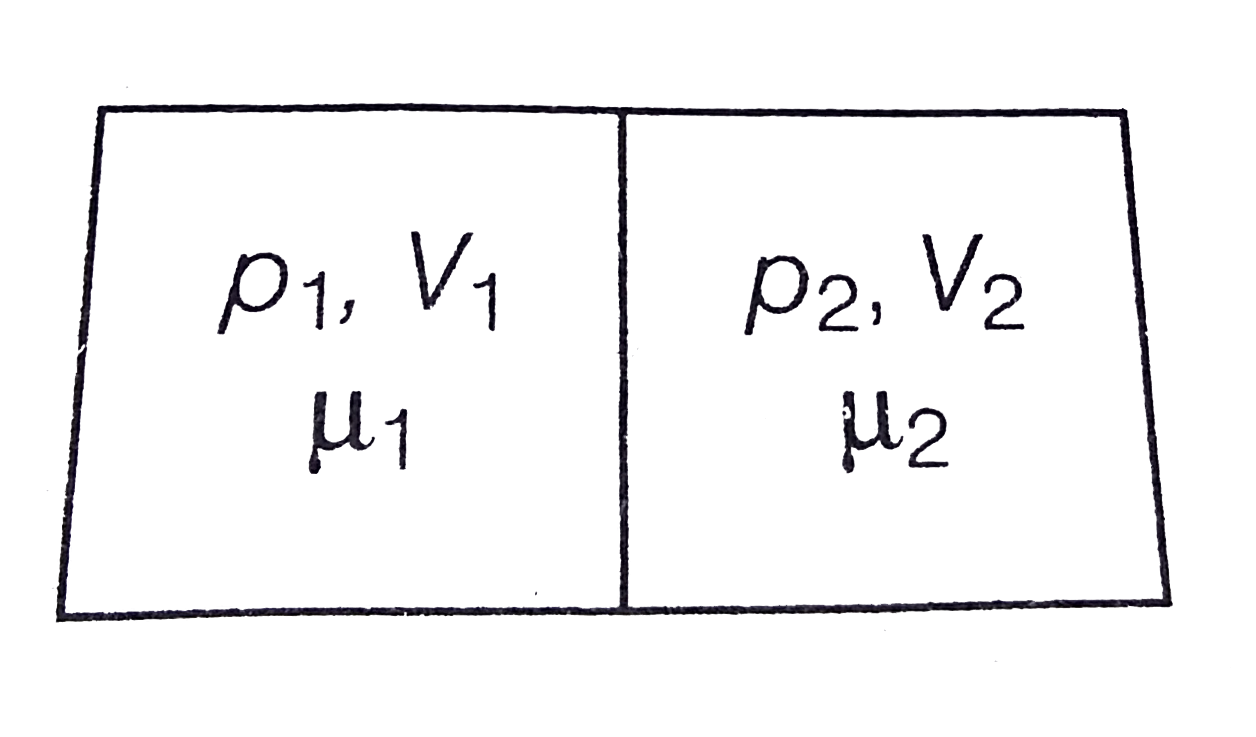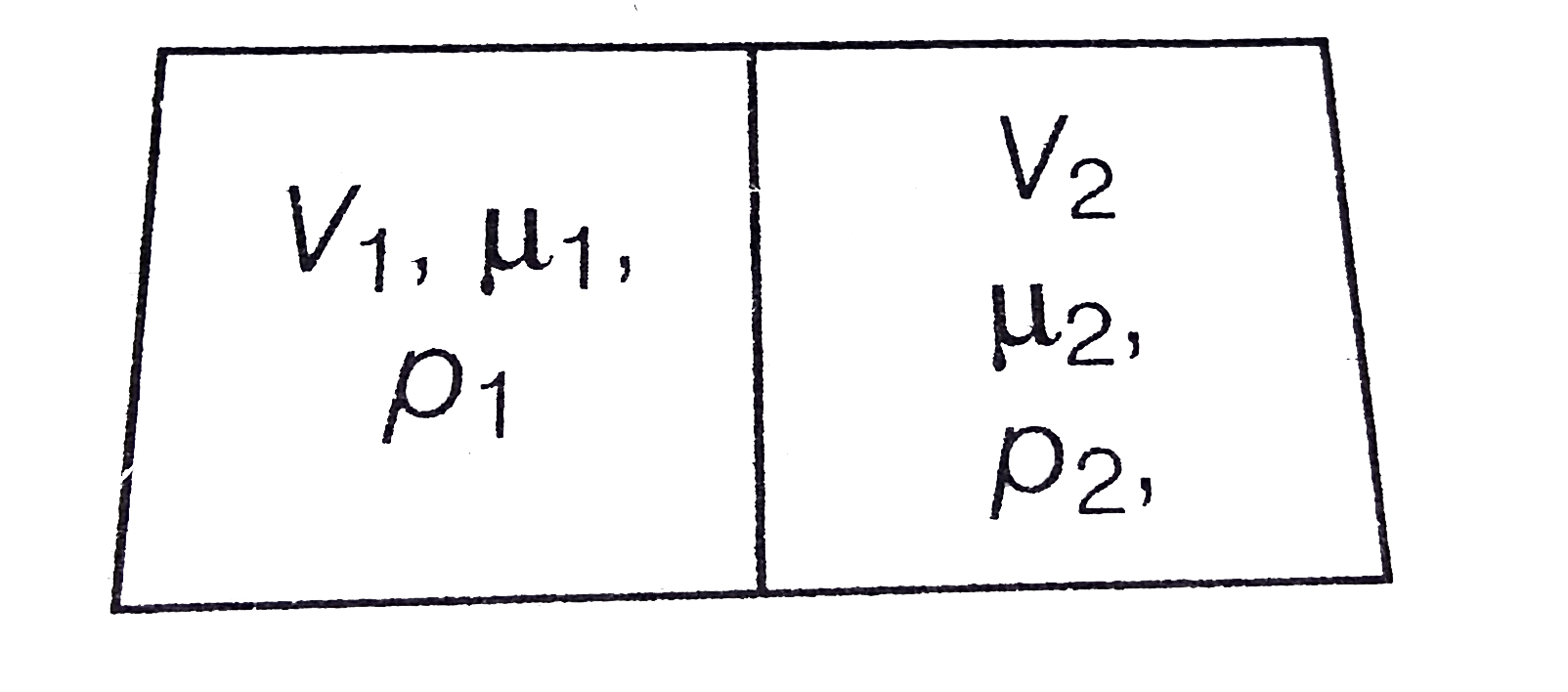Similar Questions
Explore conceptually related problems
Recommended Questions
- The container shown in figure has two chambers separated by a partitio...
Text Solution
|
- The initial volume of an ideal gas is V(1) and the initial pressure is...
Text Solution
|
- The container shown in Fig. 9(EP).6 has two chambers, separated by a p...
Text Solution
|
- If n moles of an ideal gas are expanded is isothermally and reversibly...
Text Solution
|
- An ideal monoatomic gas is initially in state 1 with pressure p(1) = 2...
Text Solution
|
- An insulated cylindrical vessel is divided into three identical parts ...
Text Solution
|
- Two chambers containing m(1) and m(2) kg of gas at pressure p(1) and p...
Text Solution
|
- If a gas of a volume V(1) at pressure p(1) is compressed adiabatically...
Text Solution
|
- The container shown in figure has two chambers separated by a partitio...
Text Solution
|