Similar Questions
Explore conceptually related problems
Recommended Questions
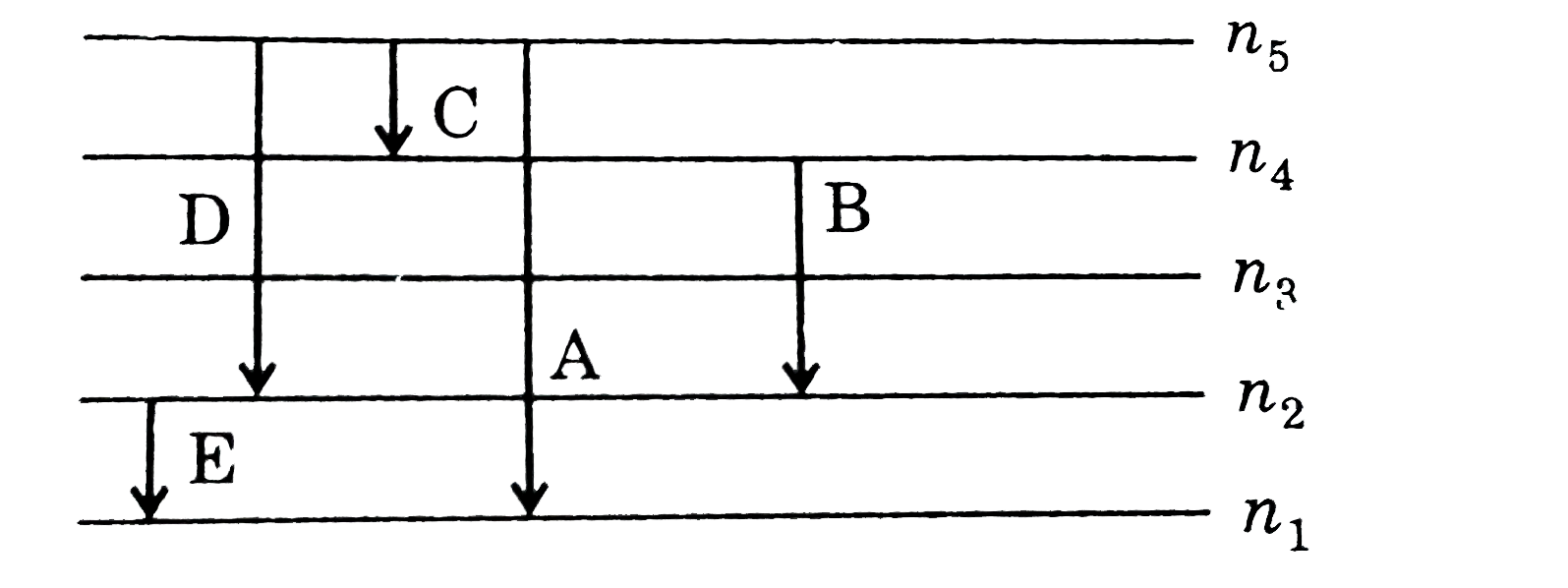
- For a H-like atom which Bohr's model some spectral lines were observed...
Text Solution
|
- For a H-like atom which Bohr's model some spectral lines were observed...
Text Solution
|
- The number of lines of Balmer series of H-atom that belong to visible ...
Text Solution
|
- Which of the following transition in He^(+) ion will give rise to a sp...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- For a hypothetical H like atom which follows Bohr's model, some spectr...
Text Solution
|
- {:("Column-I","Column-II"),("List-I","List-II"),((A)n=6ton=3("In H-ato...
Text Solution
|
- In H-atom, angular momentum of electron in certain orbit is (h)/(0.5pi...
Text Solution
|
- STATEMENT-1: A spectral lne will not be seen for a 2p(x)-2p(y) transit...
Text Solution
|