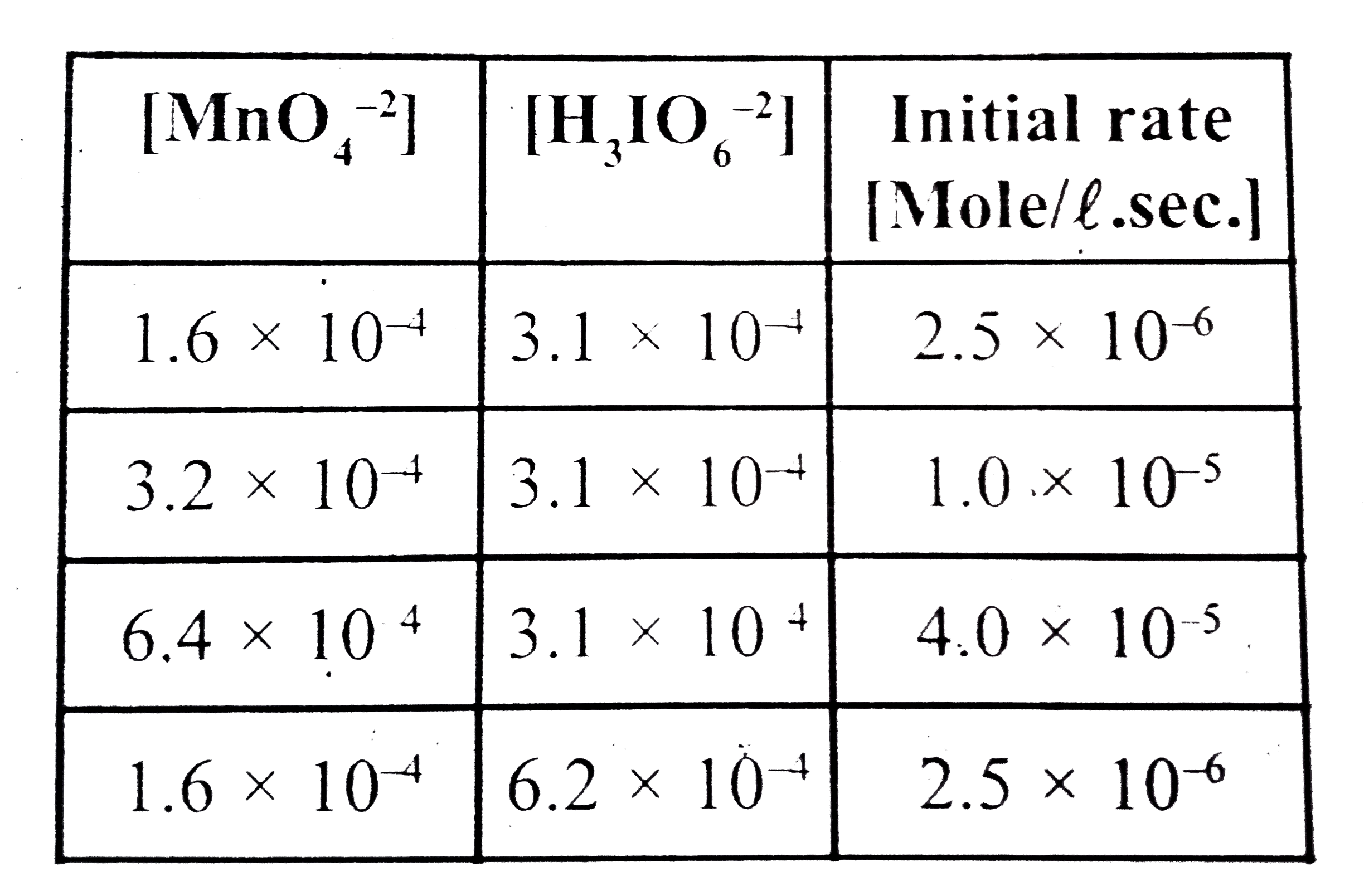A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPER 2-CHEMISTRY
- Which of the following alkene is more stable than
Text Solution
|
- The treatment of benzene with isobutene in the presence of sulphuric a...
Text Solution
|
- When phenol is treated with CHCl(3) and NaOH, the product fromed is
Text Solution
|
- For an imaginary reaction 2X+3Y to "products" Given: rate of disappe...
Text Solution
|
- Establish the rate law for the reaction : (using the data given below)...
Text Solution
|
- A gaseous reaction 2A(g) to B(g)+5C(g) shows increase in pressure from...
Text Solution
|
- The rate of a reaction increases four-fold when the concentration of r...
Text Solution
|
- The two Bronsted bases in the reaction are HC(2)O(4)^(-)+PO(4)^(3-) to...
Text Solution
|
- Phenolphthalein does not act as an indicator for the titration between...
Text Solution
|
- If pK(b) for fluoride ion at 25^(@)C is 10.83, the ionisation constant...
Text Solution
|
- Calculate the pH of solution that contains [H^(+)]=10^(-8)M is -
Text Solution
|
- Calculate the pH of 3xx 10^(-4)M Al(OH)(3) solution, if alpha(1)= 80%,...
Text Solution
|
- Which of the following resultant solution has pH =1
Text Solution
|
- 10 mL of 10^(-6) M HCl solution is mixed with 90mL H(2)O. pH will cha...
Text Solution
|
- What is the percentage degree of hydrolysis (approx.) of KCN in N/80 ...
Text Solution
|
- The pH values of 0.1 M solution of HCOONa (1), HCOOH (I), BaSO(4)(III)...
Text Solution
|
- The Ksp of PbCl(2)" is "4 xx 10^(-6). Its solublity in 0.1M CaCl(2) so...
Text Solution
|
- If 50ml of 0.2 M KOH is added to 40 ml of 0.5 M HCOOH, the pH of the r...
Text Solution
|
- H(3)PO(4)+H(2) Leftrightarrow H(3)O^(+)+H(2)PO(4)^(-),pK(1)=2.15 H3P...
Text Solution
|
- Silver ions are added to the solution with : [Br^(-)]=[Cl^(-)]=[CO(3)^...
Text Solution
|