Similar Questions
Explore conceptually related problems
Recommended Questions
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
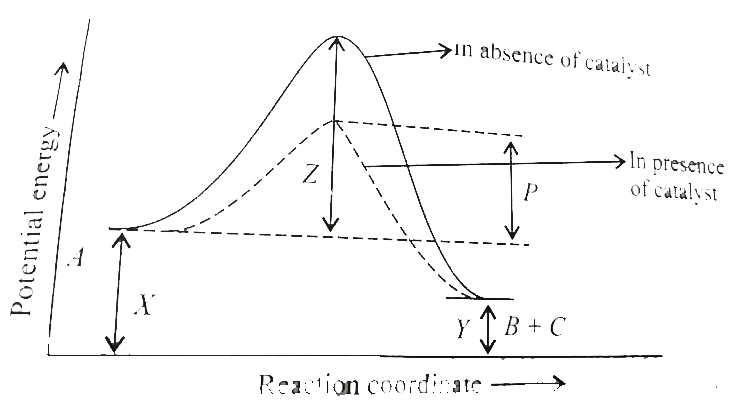
- Given the following graph. (a) Calculate Delta H for the reaction an...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Assertion : A catalyst is a substance which alters the rate of a react...
Text Solution
|
- Read the given passage and answer the questions A substance which al...
Text Solution
|
- Read the given passage and answer the questions A substance which al...
Text Solution
|