A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Level 2 Subjective|9 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY|Exercise Level 2 Single Correct|12 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY|Exercise Medical entrance gallary|30 VideosUNIT AND DIMENSIONS
DC PANDEY|Exercise Assertion And Reason|2 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES-Level 2 More Than One Correct
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition Vp^2 =...
Text Solution
|
- find the correct options.
Text Solution
|
- In the (p - V) diagram shown in figure, choose the correct options for...
Text Solution
|
- Choose the wrong options
Text Solution
|
- Along the line - 1, mass of gas m(1) and pressure is p(1). Along the l...
Text Solution
|
- Choose the correct options.
Text Solution
|
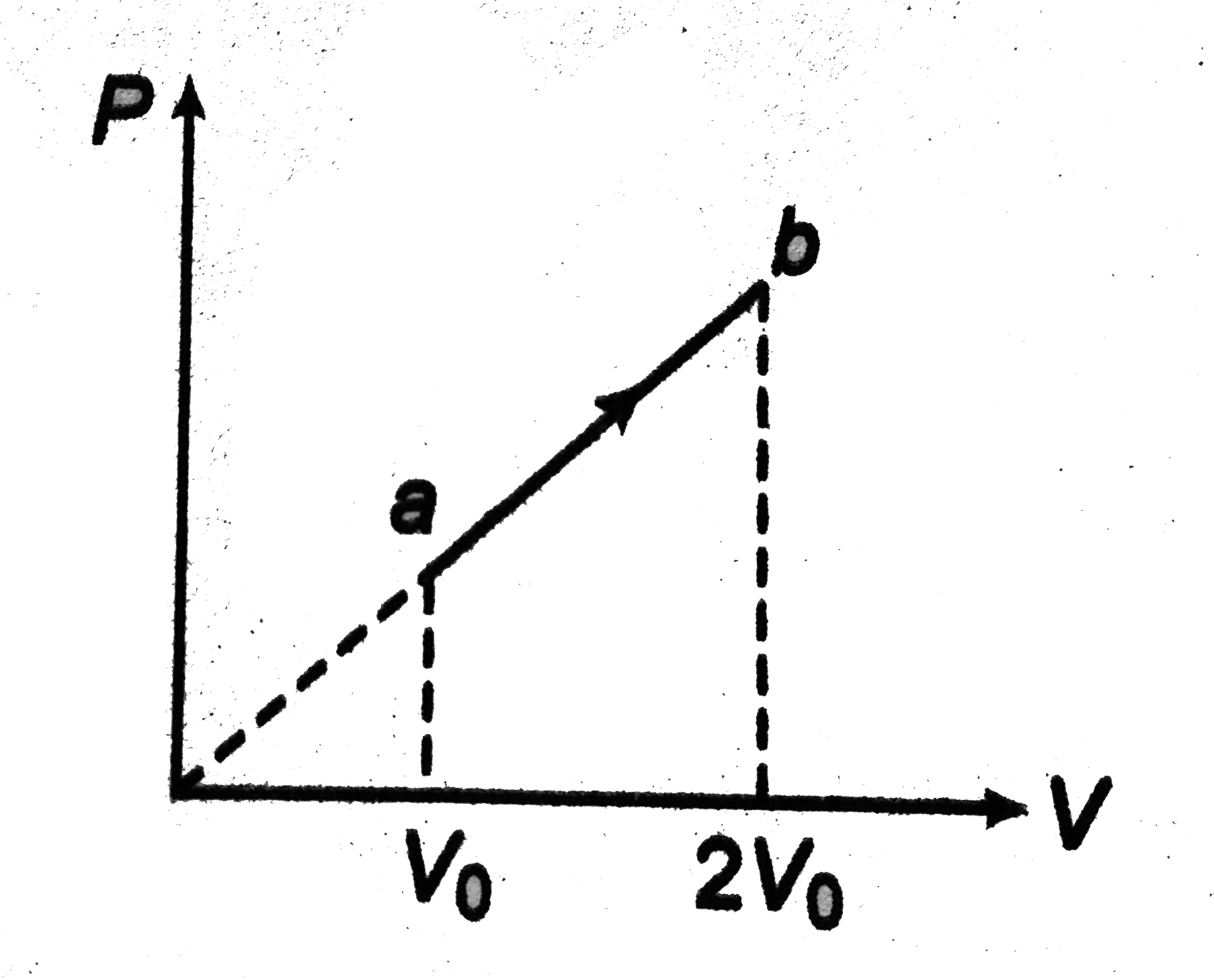 .
.