Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY|Exercise Exercise 21.4|8 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Assertion And Reason|10 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Exercise 21.2|7 VideosLAWS OF MOTION
DC PANDEY|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY|Exercise Integer type Questions|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-LAWS OF THERMODYNAMICS-Exercise 21.3
- One mole of an ideal monoatomic gas is initially at 300K. Find the fin...
Text Solution
|
- An ideal gas expands while the pressure is kept constant. During the p...
Text Solution
|
- A well insulated box contains a partition dividing the box into two eq...
Text Solution
|
- Find the ratio of (DeltaQ)/(DeltaU) and (DeltaQ)/(DeltaW) in an isobar...
Text Solution
|
- Following figure shows two process A and B for a gas. If Delta Q(A) an...
Text Solution
|
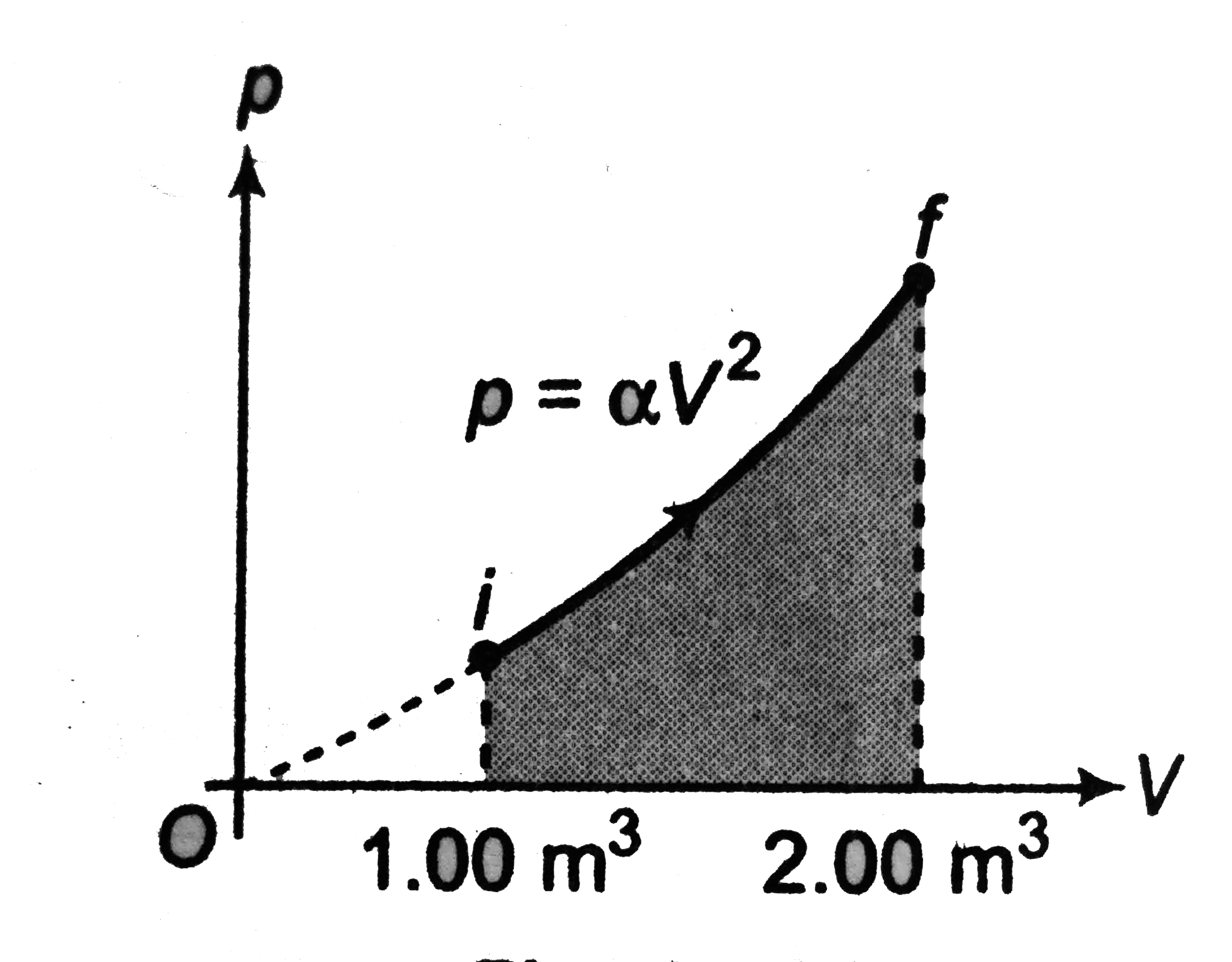
- A sample of ideal gas is expanded to twice its original volume of 1.00...
Text Solution
|
- As a result of the isobaric heating by DeltaT=72K, one mole of a certa...
Text Solution
|