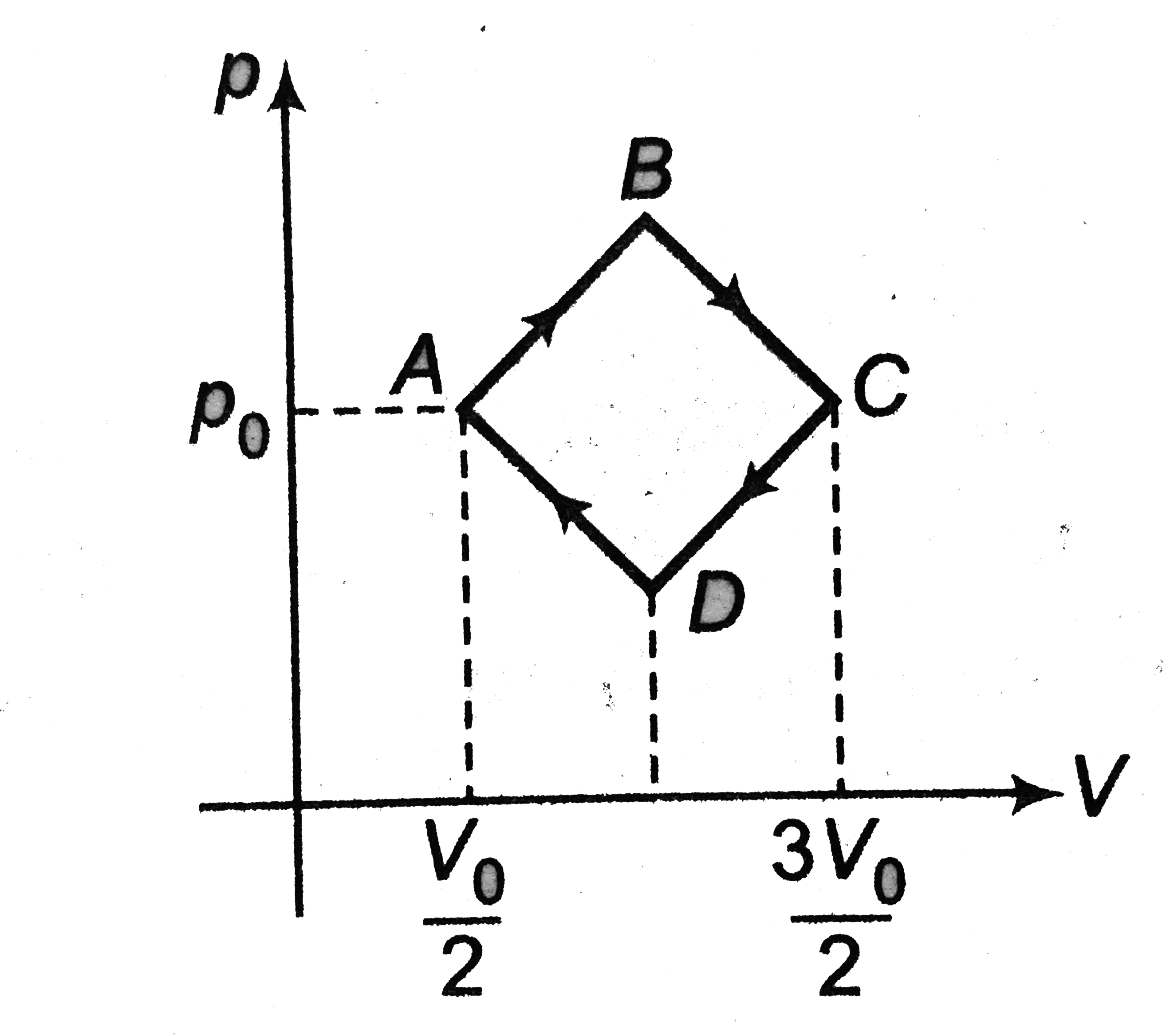
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Assertion And Reason|10 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Objective|14 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Exercise 21.3|7 VideosLAWS OF MOTION
DC PANDEY|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY|Exercise Integer type Questions|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-LAWS OF THERMODYNAMICS-Exercise 21.4
- Carnot engine takes one thousand kilo calories of heat from a reseervo...
Text Solution
|
- One of the most efficient engines ever developed operated between 2100...
Text Solution
|
- In a heat engine, the temperature of the source and sink are 500 K and...
Text Solution
|
- A Carnot engine takes 3xx10^6cal. of heat from a reservoir at 62^@C, a...
Text Solution
|
- The efficiency of a Carnot cycle is 1//6. If on reducing the temperatu...
Text Solution
|
- Refrigerator A works between -10^@C and 27^@C, while refrigerator B wo...
Text Solution
|
- A refrigerator has to transfer an average of 263J of heat per second f...
Text Solution
|
- n moles of a monoatomic gas are taken around in a cyclic process consi...
Text Solution
|