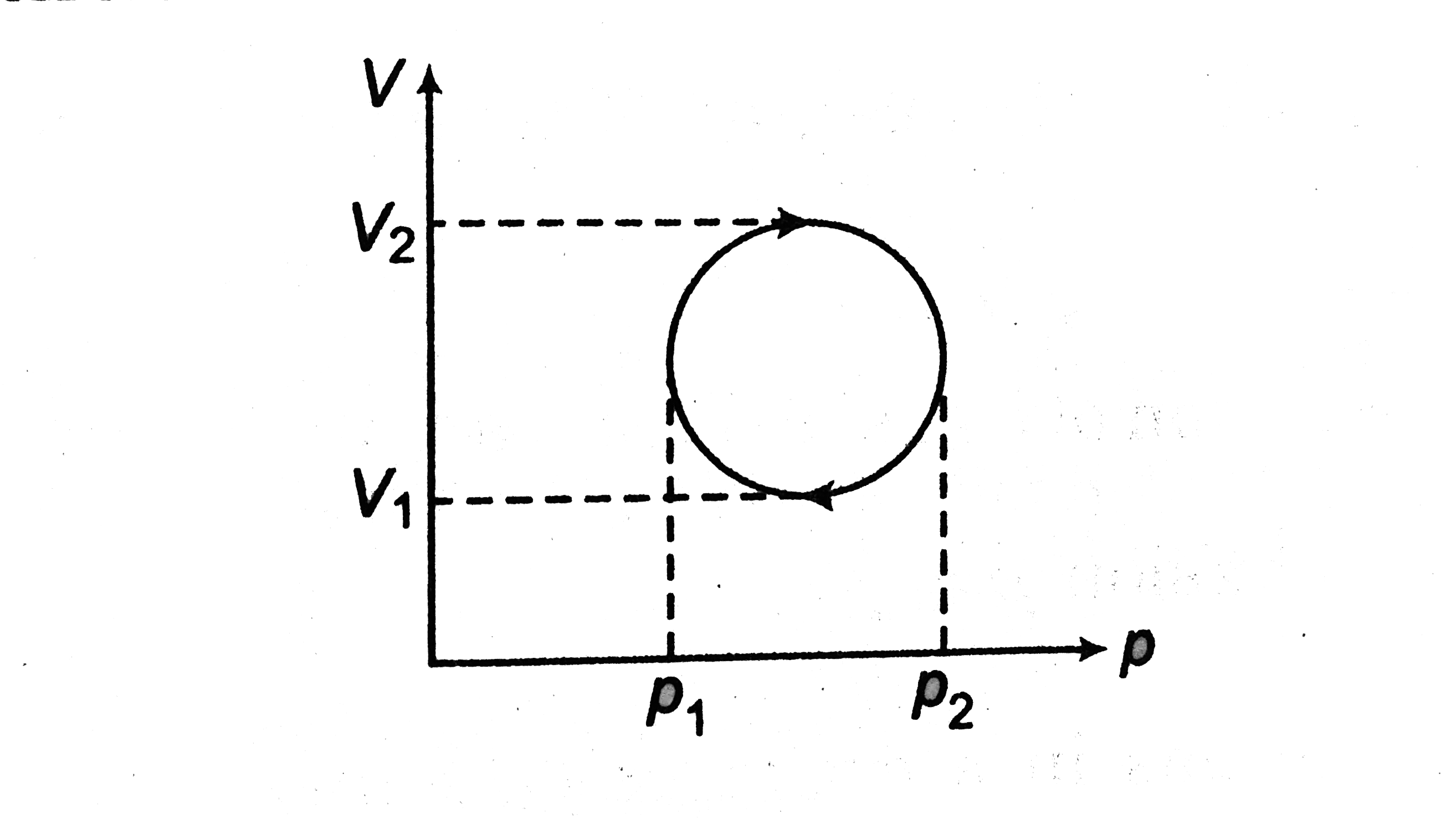
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Objective Questions|1 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Subjective|24 VideosLAWS OF THERMODYNAMICS
DC PANDEY|Exercise Level 1 Assertion And Reason|10 VideosLAWS OF MOTION
DC PANDEY|Exercise Medical entrances gallery|39 VideosMAGNETIC EFFECT OF CURRENT AND MAGNETISM
DC PANDEY|Exercise Integer type Questions|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-LAWS OF THERMODYNAMICS-Level 1 Objective
- In a process, the pressure of an ideal gas is proportional to square o...
Text Solution
|
- n moles of a gas are filled in a container at temperature T. If the ga...
Text Solution
|
- A gas undergoes A to B through three different processes 1,2 and 3 as ...
Text Solution
|
- For an adiabatic compression the quantity pV
Text Solution
|
- The cyclic process form a circle on a pV diagram as shown in figure. T...
Text Solution
|
- An ideal gas has initial volume V and pressure p. In doubling its volu...
Text Solution
|
- Following figure shows two process A and B for a gas. If Delta Q(A) an...
Text Solution
|
- A Carnot engine works between 600K and 300K. The efficiency of the eng...
Text Solution
|
- A gas is contained in a metallic cylinder fitted with a piston.The pis...
Text Solution
|
- A cycle pump becomes hot near the nozzle after a few quick strokes eve...
Text Solution
|
- In an adiabatic change, the pressure p and temperature T of a diatomic...
Text Solution
|
- A diatomic gas obeys the law pV^x= constant. For what value of x, it h...
Text Solution
|
- The molar specific heat at constant volume of gas mixture is (13R)/(6)...
Text Solution
|
- If W(ABC) is the work done in process AtoBtoC and W(DEF) is work done ...
Text Solution
|