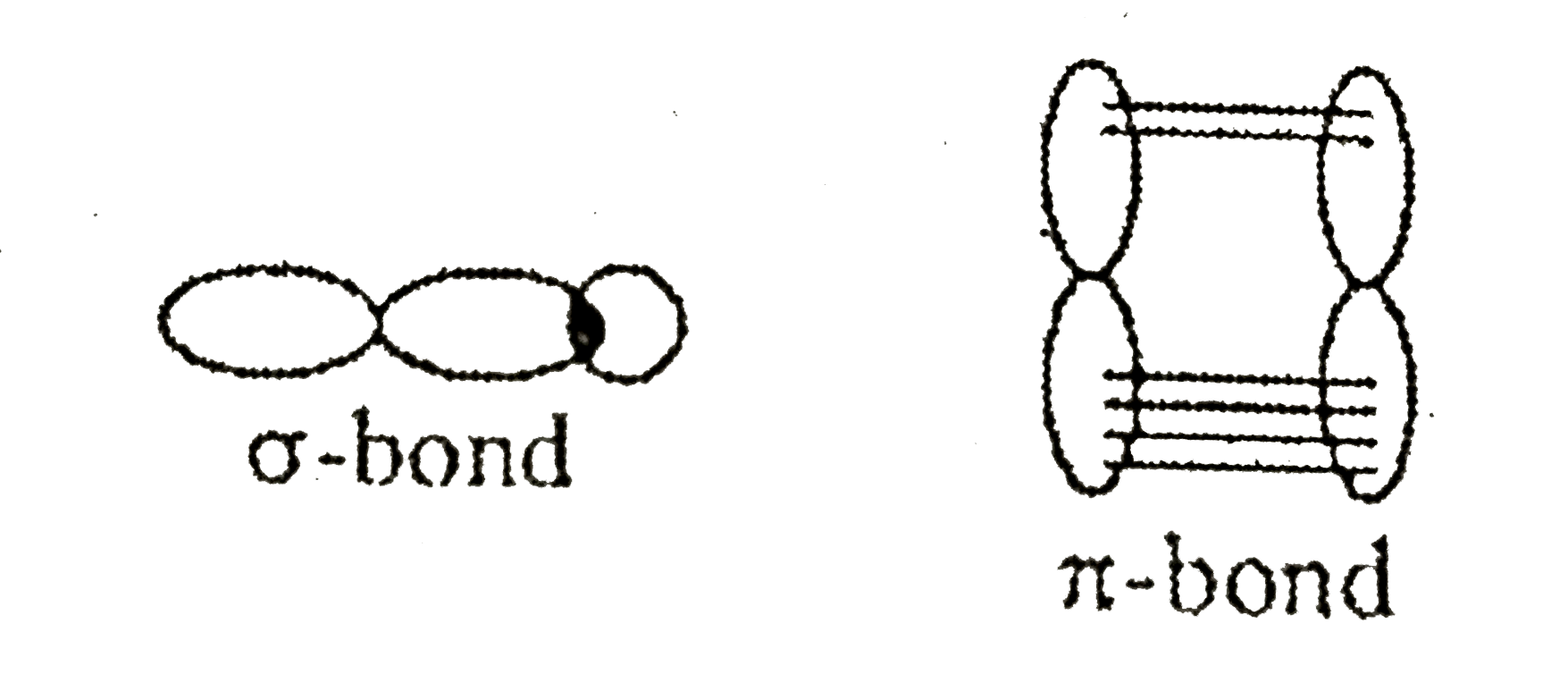A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2002-CHEMISTRY
- Assertion : During an adiabatic process, heat energy is not exchanged...
Text Solution
|
- Statement-1: Potassium and caesium are used in photo-electric cells. ...
Text Solution
|
- Assertion: Physisorption of molecules occures on surface only. Reaso...
Text Solution
|
- Assertion: Boiling and melting point of amides are higher than corresp...
Text Solution
|
- Assertion : Stannous chloride gives grey precipitate with mercuric ch...
Text Solution
|
- Assertion : DNA molecules and RNA molecules are found in the nucleus o...
Text Solution
|
- Assertion : All halogens are coloured. Reason : The halogens abs...
Text Solution
|
- Assertion : F-F bond has low bond dissociation Reason : The fluori...
Text Solution
|
- Assertion : Phenol is a weak acid than ethanol. Reason : Groups wi...
Text Solution
|
- (A) Ether behaves as bases in the presence of mineral acids. (R ) Du...
Text Solution
|
- Assertion : For Balmer series of hydrogen spectrum, the value n(1)=...
Text Solution
|
- Each question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason)....
Text Solution
|
- Assertion : Diamond is a bad conductor . Reason : Graphite is a good...
Text Solution
|
- Assertion: Atoms can neither be created nor destroyed. Reason: Under...
Text Solution
|
- Assertion: Mass and volume are extensive properties. Reason: Mass/vo...
Text Solution
|
- Assertion:Absolute values of intenal energy of substances cannot be d...
Text Solution
|
- Assertion : Cuprous ion (Cu^(+)) is colouless whereas cupric ion (Cu^...
Text Solution
|
- Assertion : Dinegative anion of oxygen (O^(2-)) is quite common but...
Text Solution
|
- Assertion : sigma-bond is strong white pi -bond is a weak bond. Reas...
Text Solution
|
- Assertion : Absorption spectrum consists of some bright lines separat...
Text Solution
|