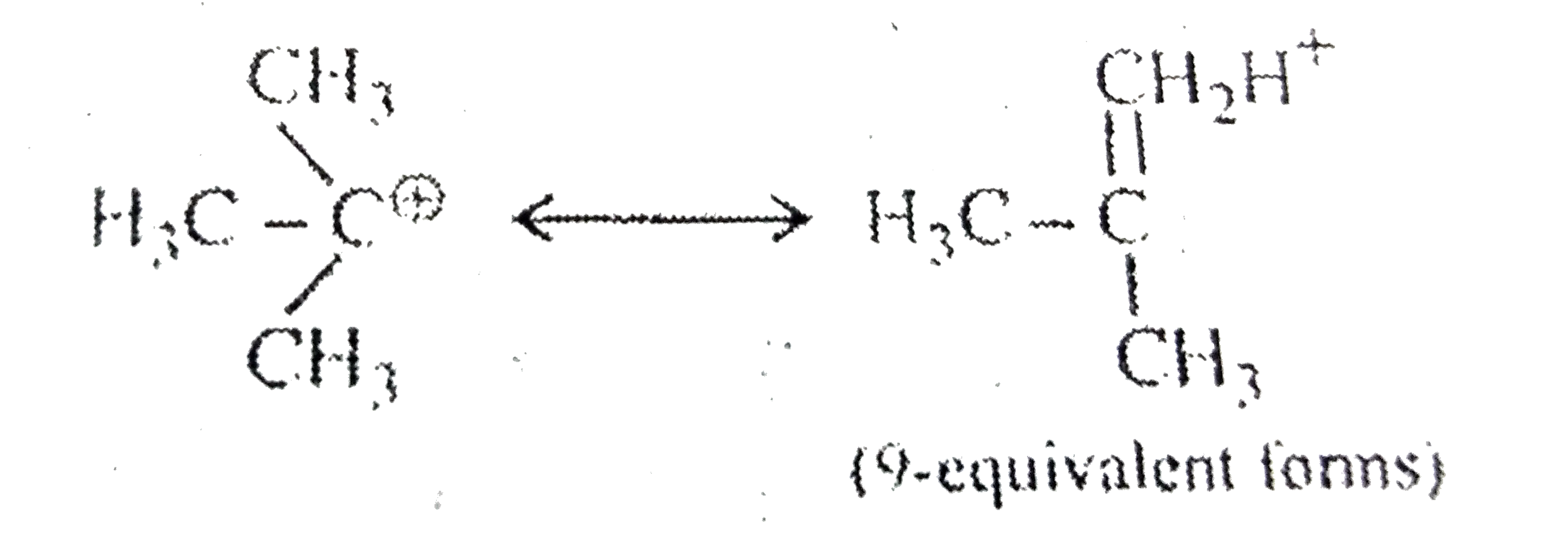
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2008-CHEMISTRY
- Which of the following is aromatic ?
Text Solution
|
- Assertion: (CH(3))(3)C COC(CH(3))(3) and acetone can be distanguished ...
Text Solution
|
- (A) Tertiary carbocations are generally formed more easily than primar...
Text Solution
|
- Assertion: If H(2) and Cl enclosed separately in the same vessel exert...
Text Solution
|
- Dalton's law of partial pressures acids to evolve chlorine. states tha...
Text Solution
|
- Assertion : According to Le-Chatelier's -principle addition of heat to...
Text Solution
|
- Assertion: Cyclohexane exhibits keto-enol tautomerism. Reason: In cy...
Text Solution
|
- Phenol is more reactive than benzene towards electrophilic substitutio...
Text Solution
|
- Assertion (A): May endothermic reactions that are not spontaneous at ...
Text Solution
|
- Assertion: Benzaldehyde is more reactive than ethanal towards nucleoph...
Text Solution
|
- Assertion: Bleaching powder reacts with dilure acids to evolve chlorin...
Text Solution
|
- Assertion : Teflon has high thermal stability and quantuin number. che...
Text Solution
|
- Assertion : In high spin situation, configuration of d^5 ions will ...
Text Solution
|
- Assertion (A) : Cu gets readily corroded in acidic aqueous solution. ...
Text Solution
|
- Assertion : When a connection solution is diluted by acidic aqueous so...
Text Solution
|
- Assertion : Anilinium chloride is more acidic towards electrophilic su...
Text Solution
|
- Assertion : Pyrrole is an aromatic heterocyclic compound Reason : ...
Text Solution
|
- Assertion : 2-Butanol on heating with H2SO4 gives 1-butenol on heating...
Text Solution
|
- Asseration: SeCl(4), does not havea tetrahedral structure. Reason: S...
Text Solution
|
- Assertion : Liquid NH3 is used for refrigeration Reasong: Liquid ...
Text Solution
|