A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2010-CHEMISTRY
- When 25 g of Na(2)SO(4) is dissolved in 10^(3) Kg of solution, its co...
Text Solution
|
- Degree of unsaturation of A = 2, it contains no double or triple bonds...
Text Solution
|
- The shape and hybridisation of some xenon oxyfluorides are given. Choe...
Text Solution
|
- The standard half-cell reduction potential for Ag'|Ag is 0.7991 V at 2...
Text Solution
|
- Which of the following acids will not evolve H(2) gas on reaction with...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Stomach acid, a dilute solution of HCl in water, can be neutralized by...
Text Solution
|
- For the electrochemical cell, (M)|M^(+))||(X^(-)|X). E^(@)(M^(+)//M)...
Text Solution
|
- Which is optically inactive?
Text Solution
|
- Assertion : Magnesium is extracted by the electrolysis of fused mixtur...
Text Solution
|
- Assertion (A) : The equilibrium constant is fixed and characteristic f...
Text Solution
|
- Assertion : PCl(5) is covalent in gaseous and liquid state but ionic i...
Text Solution
|
- Statement-1: Zinc displaces copper from copper sulphate solution. St...
Text Solution
|
- Assertion : underset("-carbethoxy- 2-butenoic acid.")(CH-underset(COOC...
Text Solution
|
- Assertion: Helium has the highest value of ionisation energy among all...
Text Solution
|
- Assertion : The nuclear isomers are the atoms with the same atomic num...
Text Solution
|
- Assertion : Conductivity of silicon increases by doping it with group-...
Text Solution
|
- Assertion : The overall order of the reaction is the sum of the expone...
Text Solution
|
- Assertion : Transition metals are poor reducing agents. Reasons : Tr...
Text Solution
|
- Assertion : Aldol condensation can be catalysed both by acids and base...
Text Solution
|
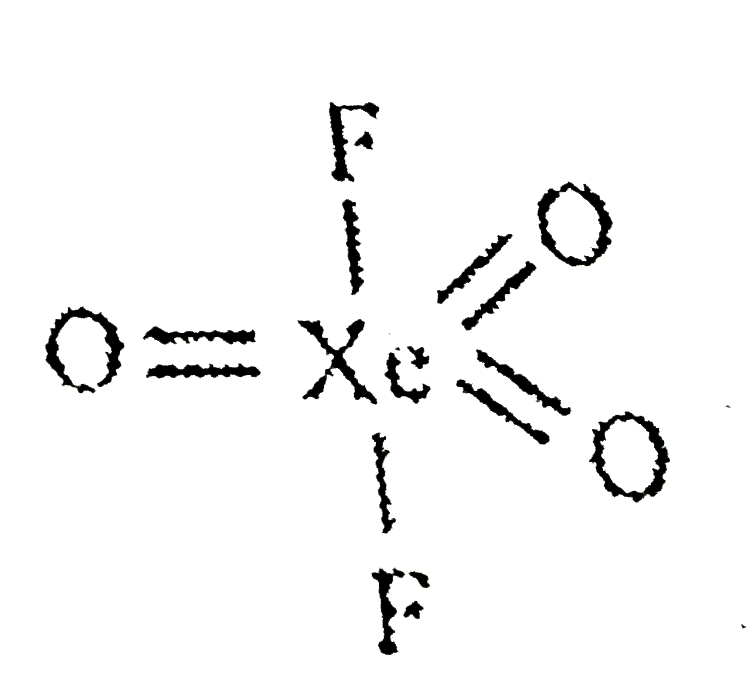 No. of lone pair of Xe=0 and no. of bond pair =5 Hybridisation of `Xe=sp^(3)d`
No. of lone pair of Xe=0 and no. of bond pair =5 Hybridisation of `Xe=sp^(3)d`